
Two Grizzly Bears playing at the Bronx Zoo. One of the bears, Jughead (all the Bronx grizzlies are named after “Archie” characters) died earlier this year.

Two Grizzly Bears playing at the Bronx Zoo. One of the bears, Jughead (all the Bronx grizzlies are named after “Archie” characters) died earlier this year.

One of Charles R. Knight’s paintings of Smilodon fatalis, this one menacing a giant sloth stuck in tar (off panel).
There are few fossil mammals that are as impressive the saber-toothed cat Smilodon fatalis, but despite it’s fearsome dentition some recent new reports have suggested it was more of a pussycat when it came to bite strength. This seems to be counter-intuitive; how could such an impressive animal be associated with the term “weak”? Part of it has to do with word choice, but the larger issue has to do with the fact that the bite of Smilodon wasn’t as strong as that of some other carnivores (extinct and extant), as well as dentition and feeding ecology. This issue goes far beyond just one genus or species, however, as Smilodon was only one of many genera that bore massive canines. In fact, huge “sabers” have evolved over-and-over again in the mammalian lineage (see this post and also this post for information about the cat-like ones), including the famous fangs of the machairodontine felids (saber-toothed cats) and their look-alike nimravid relatives.
Lateral, anterior, and dorsal views of the herbivore Uintatherium (Note the prominent canines). From Marsh, O.C. “The Fossil Mammals of the Order Dinocerata.” The American Naturalist, Vol. 7, No. 3. (Mar., 1873), pp. 146-153
Skull of another member of the Dinocerata; “Loxolophodon cornutus” (today known as Eobasileus cornutus). Again, note the prominent canine. From Cope, E.D. “The Amblypoda (Continued).” The American Naturalist, Vol. 19, No. 1. (Jan., 1885), pp. 40-55.
Although this post will primarily be concerned with the great “sabercats,” large, dagger-like canine teeth having been evolved multiple times by many different unrelated animals during the course of life on earth. In some herbivorous creatures, like the extinct Uintatherium and even in the extant Musk Deer, the fangs reflect sexual dimorphism and probably sexual selection, but the sharp teeth don’t seem to have a prominent function in mastication or processing of food. Likewise, large canine teeth are present in living baboons (Papio sp.), and the sexual dimorphism exhibited between the dental equipment of the males and the smaller canines of the females has long been noted (males often yawn to show off their canines, the size of their teeth being very intimidating indeed). Do the same considerations of sexual selection and dimorphism hold true for the saber-toothed cats, too? Unfortunately, fossil evidence does not always allow comparisons of the two sexes, but extant big cats and some death-trap sites have provided some information to work with. From Salesa, et al. (2006);
Among the Carnivora, sexual dimorphism is more marked in canine size than in other dental features or skull size, and these differences can be related to the breeding system. Species in which a male defends a group of females tend to be more dimorphic than those with monogamous pairs or groups of males and females. Felids are dimorphic animals, but mainly in reference to body size, with the mane of male lions being a unique example of morphological variation between sexes among the family.
This makes sense; if a male keeps a harem of females and has to defend it from other males, the species is more likely to exhibit sexual dimorphism than not. In cats, however, it seems to be more about body size (and possibly characters that wouldn’t fossilize in extinct species) than about tooth size (which would serve important other functions, so any sexual selection would be mitigated by natural selection), although we can’t be sure of this being that there are no living sabercats to study. Personally, I think there could be a sexual-selection component in some groups, but the saber-canine is so prominent in so many extinct felids and nimravids that it is extremely doubtful that all the lineages converged on similar tooth structures because of sexual selection/dimorphism, the functional advantage of larger teeth likely coming first. A lack of sexual dimorphism when considering morphology as a whole, however, may suggest a more solitary lifestyle where territories may or may not overlap are maintained and direct competition for females is not as fierce, especially since the females move through territories rather than living with a male. Such a strategy may have been employed by the late Micoene sabercat Paramachairodus ogygia. Salesa, et al. (2006), working with an assemblage made up of many of the more basal felids, have even been able to come up with a hypothesis about life history of the ancient animals based upon their finds in Spain;
[T]he probable territorial behaviour for Par. ogygia would be very similar to that of jaguars, in which males defend large, overlapping territories that include smaller territories of several females. This model is similar to that of the leopard, but in this species male territories never overlap, which could explain the different sexual dimorphism index of this species with respect to Par. ogygia and jaguar…
So, if Par. ogygia behaved more like jaguars and leopards than lions, the presence of juveniles in the trap would be highly improbable, as is the case. But in addition to the scarcity of juveniles, the sample from Batallones-1 has another interesting feature: it is mostly composed of young adults, that is, individuals with the complete permanent dentition, but without any trace of wear. These animals, which would have recently become independent of their mothers, would not as yet have had any territory, moving instead through the ranges of other adults and being more easily attracted by an easy meal, such as carrion. This age distribution therefore suggests that the sample of Par. ogygia trapped in Batallones-1 corresponds to that fraction of non-resident young individuals, both males and females, which were in a phase of dispersion. In the case of leopards, such individuals are more daring – or less cautious – than adults, and they have been seen crossing rivers in spate, whereas resident adults only cross at times of lower water. It has also been noticed that among these individuals, males are even more inclined to make these incursions than females, which remain longer with the mother, especially if there is good availability of food. If this pattern of dispersion behaviour applied to the young adults of Par. ogygia, it is likely that they were trapped in Batallones-1 more often than the resident adults.
Saber-Toothed Felid and Nimravid diversity (click for a larger image). From Emerson, S.B., and Radinsky, L. “Functional Analysis of Sabertooth Cranial Morphology.” Paleobiology, Vol. 6, No. 3. (Summer, 1980), pp. 295-312.
While the life histories of extinct mammalian carnivores are interesting in and of themselves, it is the teeth and terrifying bite of the sabercats that we are most concerned with here. Smilodon is the celebrity of saber-toothed cats, but the fossil record preserves a wide diversity of carnivores with large canine teeth, and even within the larger groupings there are even more subdivisions, the skulls of saber-toothed felids being widely variable. As discussed in the background material, nimravids are saber-tooth look-alikes that diverged from a common ancestral line earlier than the carnivores that would give rise to Smilodon, but the two lines are still closely related and have undergone parallel evolution. There is still some reshuffling of taxa going on and the true evolutionary history/affinities of many of the forms is still being worked out, but most forms you’re likely to see grouped together at a museum fall into either the nimravid or felid camps. The focus of this essay, however, will be on felids, and although they are often discussed along with their nimravid cousins the larger amount of work has been done on the felids and so we must leave the nimravids.
With the felids, then, there seem to be three kinds of sabercat that hint at differing predatory tactics, prey, and habitat. Indeed, evolution did not create carbon copies of the same creature, barring life from becoming adapted to varying circumstances; there is more variety than would be first assumed if we based all our research on the presence of prominent canines. Instead, there seem to be three “ways of being” a saber-toothed cat, as outlined by Martin, et al.;
Saber-toothed carnivores… have been divided into two groups: scimitar-toothed cats with shorter, coarsely serrated canines coupled with long legs for fast running, and dirk-toothed cats with more elongate, finely serrated canines coupled to short legs built for power rather than speed. In the Pleistocene of North America, as in Europe, the scimitar-cat was Homotherium; the North American dirk-tooth was Smilodon. We now describe a new sabercat from the Early Pleistocene of Florida [Xenosmilus], combining the scimitar-tooth canine with the short, massive limbs of a dirk-tooth predator. This presents a third way to construct a saber-toothed carnivore.
Xenosmilus hodsonae, Homotherium cf. crenatidens, and Homotherium serum. From Martin, L.D., Babiarz, J.P., Naples, V.L., and Hearst, J. “Three Ways To Be a Saber-Toothed Cat.” Naturwissenschaften, Vol. 87, No. 1 (Jan. 2000), pp. 41-44
As Martin notes, there appears to be a number of adaptational “trade offs” that sabercats in North America and Europe were subject to; fast-moving gracile forms had shorter sabers, but stouter and more powerful forms had the longer, more laterally flattened canine teeth. The “third way” that combined characters from both groups was exemplified by Xenosmilus (which Martin, et al. say would have seemed more like a bear than a cat, despite actual evolutionary relationships to the contrary). Still, leaving the overall structure of the body aside for a moment, the arrangement and sizing of the teeth of the different groups can be very telling. Martin, et al. again lay out what the usefulness of the differing tooth arrangements;
When biting, the long sabers of dirk-toothed cats may have cut parallel slits for some distance before the relatively smaller incisors could be applied. In scimitar-toothed cats the shorter canines and longer incisors worked more as a unit, first cutting parallel slits with the canines, immediately followed by the incisor arc removing the strip of flesh. Such a large open wound would have bled profusely, traumatizing the victim. If the incisors and canines acted in unison, the torsional forces on individual teeth would have been reduced, resulting in fewer restrictions on bite placement. In felids the size of the sagittal crest is directly proportional to the forces exerted by the temporalis musculature. Scimitar-toothed cats have a sagittal crest that is generally less pronounced than that in their dirk-toothed contemporaries. In a modification of the typical scimitar-tooth condition, the new cat from Florida exhibits both an elongated sagittal crest and an enlarged temporalis muscle that would have permitted a stronger bite.
While such a passage might not seem significant at first, it shows that there is more going on in a sabercat’s skull that is important to biting than just the size or shape of the canines. The placement of the incisors, for instance, seem to make a difference in biting strategy and force, dirk-toothed cats like Smilodon exhibiting a condition where the incisors are out forward of the canines. When this is taken into account, as well as the length of the canines, it seems that the canines would slash for quite some distance before the incisors could be used at all in comparison to the scimitar-toothed sabercats, the placement of the incisors in scimitar-tooths seemingly strengthening the biting teeth at the front of the jaw. The sagittal crests of these creatures should also be taken into account, such structures giving students of paleontology an indication of how carnivores (or herbivores, in the case of gorillas) have been adapted to achieve higher bite forces. Such ridges atop the skull for muscle attachment are not unique to sabercats, however, and there are some animals that have taken the structure to even greater extremes;

The extinct “bear dog” Amphicyon at the AMNH. Note the size of the sagittal crest, the reduction of the bony enclosure around the eyes, and the large holes on the side of the skull for jaw muscle attachment.

The extinct “saber-toothed” creodont Hyaenodon at the AMNH. Again, note the sagittal crest, reduction of bone enclosure around the eye, and the large canines.

The skull of the nimravid Hoplophoneus on display at the AMNH. Note the size of the canines and sagittal crest in comparison with Hyaenodon and Amphicyon.

The skull of Smilodon on display at the AMNH.

The skull of the marsupial predator Thylacoleo at the AMNH. Note the large openings on either side of the skull for the jaw muscles.

Ventral view of the skull of Thylacoleo. From E.D. Cope’s “The Tertiary Marsupialia” in The American Naturalist, Vol. 18, No. 7. (Jul., 1884), pp. 686-697.
Looking at the various groups, all show adaptations that increase the amount of available muscle attachment to achieve more powerful bites, modifying the skull in two ways. First, a sagittal crest (as already discussed) is often present to some degree, often being greater in omnivores or bone-crushing carnivores as they require greater forces to crack hard foods (although recent research by Wroe, et al. suggest that bone crushers like Spotted Hyena might not have the highest bite forces). Likewise, the holes between the skull and cheek bones are often enlarged or widened (the extreme of this group being Thylacoleo), the more muscle that can pass from lower jaw to skull being directly correlated to bite strength. What is interesting about sabercats, when considering these factors, is that they seem to be in the middle. They don’t exhibit adaptations of the skull to the extreme as in Amphicyon or Thylacoleo, but they still exhibit changes allowing for powerful bites (strong enough to kill and consume prey, at least). The trend is obvious and has not been missed by reseachers, and Emerson says the following about it;
With enlargement of upper canines, skulls of paleofelid, neofelid, marsupial and, as far as the record shows, creodont sabertooths were remodeled in similar ways. This evolutionary convergence in cranial morphology is not surprising, since most of the modifications relate to allowing increased gape while retaining bite strength at the carnassial. Those are factors essential for all sabertooths, and the possible ways to achieve them, starting from a generalized mammalian cranial morphology, are limited…
Why did sabertooth specializations evolve so many times? Their multiple evolution, plus the fact that several species of sabertoothed felids existed for most of the history of the family (from about 35 Myr to about 15,000 yr BP) suggest that sabertooth canines provided an effective alternative to the modern carnivore mode of killing prey

The skull of the saber-toothed cat Megantereon. Like in Smilodon, not how the incisors jut out (as well as the overly large nasal opening in this genus).
The basic mechanics of the skull just discussed gives researchers clues as to how sabercats could have killed their prey, but reconstructing ancient predator/prey interactions with no exact modern equivalent is difficult. Indeed, debate has gone on for years as to how sabercats used their teeth to bring down prey (see Simpson’s paper), either by stabbing, cutting, slicing, or even (as silly as it may seem) by crushing. What does seem apparent today, however, is that the canines of the sabercats were relatively delicate, and it would be unwise to fully sink them into a struggling animal as they may easily be broken off. Even if such an attempt to deeply puncture a prey item was not undertaken, biting full-force into bone could have also easily damaged teeth (or even broken them off), making it unlikely that sabercats jumped onto the back of their prey and tried to sink their teeth into the back of the prey’s skull like some modern cats. Recent research has even shown that the skull of Smilodon was ill-suited to handle stresses associated with struggling prey when compared to the skull of a lion, and I wonder how often individual Smilodon perished because of stresses associated with taking down prey if the victim was not brought down and killed quickly. Indeed, it seems that the long teeth were better suited to slicing soft flesh, i.e. cutting open the belly of prey or slicing open the throat, rather than piercing rough hides and ramming through bone.
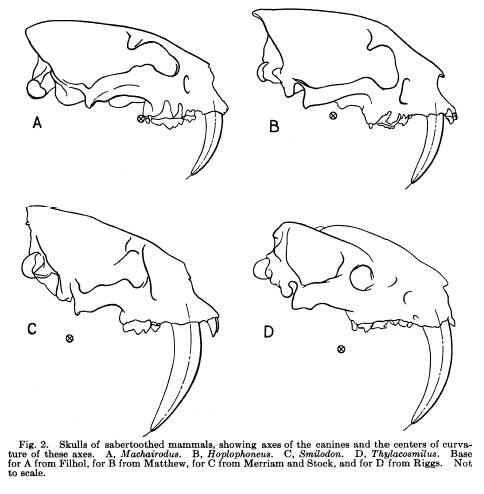
Skulls (mandibles not pictured) of 4 “saber-toothed” mammals from “The Function of Saber-Like Canines in Carnivorous Mammals” by G.G. Simpson, American Museum Novitiates, August 4, 1941. Pictured are A) Machairodus (felid), B) Hoplophoneus (nimravid), C) Smilodon (felid), and D) Thylacosmilus (marsupial).
As just discussed in terms of tooth and skull stressed, many factors of life history, behavior, and morphology of extant big cats and sabercats might be similar, but the massive canines of the extinct group seem to infer a different killing strategy, and there is no reason to assume that they were like modern big cats in every respect. Salesa, et al. sums it up this way;
Extant felids kill small animals by biting on the nape or directly on the skull, using their rounded-section canines, but if any sabre-toothed cat tried to do this they would have risked breaking the laterally flattened upper canines. For this reason, it is more probable that they developed some behavioural mechanism to minimize that risk, such as ignoring prey below a given size. It is likely that machairodontines developed this ethological trait early in their evolution, and so narrowed their prey size range in comparison with that of felines, which hunt both large and small animals. This high specialization has been pointed out as one of the possible reasons for the gradual decline and final extinction of the sabre-toothed cats in the Pleistocene… The development of this strategy was probably the key reason for the sabre-tooted cats becoming the dominant predators in the land mammal faunas from the Late Miocene to Late Pleistocene.
It might not immediately make sense that felids with fragile teeth would specialize in eating large prey, but that is whale the fossil evidence (as we currently understand it) infers. While the smallest prey would pose no problems (outside of not being a fully satisfying meal), but medium sized prey with smaller areas of soft flesh (like the stomach and neck) would potentially be more dangerous and a more exact bite would be needed to prevent damage to the teeth and skull. Hence, it seems that the slashing and ripping of soft tissue in larger animals was the main method of killing prey (after it had been brought down or slowed by blood loss), taking hypercarnivory to an even more specialized extent.

An Amur Leopard yawns. Note the relatively small (but still fearsome) canines of the upper and lower jaw.
What, then, of a smaller living cat, the Clouded Leopard (Neofelis nebulosa and N. diardii), which has been heralded as a modern analog of sabercats? As Christiansen notes, Clouded Leopards are a bit bizarre, and it is incorrect to call them “small” big cats or modern sabercats, the genus showing a number of convergences with extinct forms while remaining distinct from the famed genus Panthera;
The skull morphology of the clouded leopard sets it apart from other extant felids, and in a number of respects it approaches the morphology of primitive sabertooths. This indicates convergence of several characters in machairodontine felids and the clouded leopard, mainly as adaptations for attaining a large gape. This raises doubts about the characters hitherto considered as distinguishing sabertoothed from nonsabertoothed predators…
Clearly, Neofelis and the sabertooths independently evolved a suite of the same specializations for the same overall purpose of attaining a large gape, a prerequisite for efficient jaw mechanics with large canines, but the reasons for evolving these characters need not have been similar. Based on analyses of lower jaw bending moments and inferred resistance to mechanical loadings, Therrien (2005) suggested that Neofelis could be at the beginning of a new sabertooth radiation. Such claims are difficult to test, however, since the extant sister taxon to Neofelis (Panthera) shares none of its sabertoothed characters, and the fossil record provides no clues of felids closer to Neofelis than Panthera. At present, however, there is little evidence to suggest that Neofelis can be regarded as an “extant sabertooth,” although it clearly shares a number of characters with them that are absent in other extant felids. On the other hand, it cannot be regarded as simply an intermediate between large and small felids, as normally assumed. The presence to some extent of characters normally ascribed to sabertooths in Neofelis raises doubts about their functional and evolutionary significance in primitive machairodonts such as Nimravides or Paramachairodus, hitherto the only reasonably well-known primitive machairodont. Such animals need not have shared the presumed functional skull morphology of later, more derived sabertooths and are perhaps not to be regarded as “sabertoothed” at all, if by sabertoothed is implied animals functionally significantly different from extant felids.
Again, this shows a convergence of functional morphology despite existing evolutionary relationships, many felids being adapted in similar ways. As stated previously, the large canines of saber-toothed predators required the animals to open their jaws wide but also narrowed their predatory niche to some extent. Likewise, various tests seem to show that the bite of sabercats like Smilodon was “weak,” with news reports often relating that the terrible felids were more like big housecats when compared to living big cats. This is a mistake (and it would be a grave one for anyone ever to cross a sabercat), born of a lack of recognition that bite forces exist on a continuum and are related to a number of factors and cannot simply be deemed “weak” or “strong” without further comment. Christiansen relates the bite force of Smilodon as such;
[A]lthough large sabertooths such as Smilodon and Homotherium had weaker bite forces than lions or tigers, their bite forces were broadly comparable to those of jaguars and large leopards, and, thus, cannot be claimed to have been “weak”. Lower bite forces at any given body size were probably evolutionarily possible owing to a marked contribution from the upper cervical musculature to the killing bite, which… was absent in Neofelis and primitive machairodonts such as Paramachairodus. Thus, bite force analysis may constitute a hitherto overlooked parameter in evaluating whether or not primitive machairodonts such as Paramachairodus or Nimravides really did employ a canine shear bite with a marked contribution from the cervical muscles to subdue prey, or killed in a manner similar to extant felids, which requires a stronger killing bite…
In many Plio-Pleistocene communities predator competition was more severe than today, and a sabertooth killing mode could be a way of ensuring faster kill rates, since a throat shear-bite most likely would kill prey faster than a throttling throat bite, common in extant pantherines. In lions, it can take up to 13 minutes to kill large prey, and in such cases the prey is frequently killed by disemboweling by other pride members. In the cheetah a suffocation bite can take even longer to kill prey. Carcass theft and feeding competition is very common among extant large, sympatric predators, and a faster kill mode could be a way of reducing the risk of carcass theft from competing predators. In many large predators with sympatric competitors, rapid consumption can be a way of reducing the risk of carcass theft, and this would most likely have been accentuated in past ecosystems with more intense large predator competition. Accordingly, the morphology and behavior of extant predators need not reflect the circumstances to which they became adapted when they evolved. More intense competition could accelerate the evolution of a sabertooth morphology…
This passage reflects the problems with reconstructing bite forces and predation techniques of extinct creatures; more is involved than just the opening and closing of the jaw. The neck muscles of many sabercats (except in some of the more basal members, as noted) likely contributed to the strength of the bite in a way that’s not directly testable today. Likewise, the killing technique of sabercats might not have required a bite as strong as a modern-day tiger, as in a land filled with other predators, it might simply take too long to try and suffocate a prey animal or bite through the back of their skull. Disemboweling or tearing out the throat of the prey item, by contrast, is a much quicker way to do large amounts of damage but it seems that it would require teamwork, solitary extant big cats often opting for a killing neck bite when the prey has been brought down. Even if this is eventually shown to be incorrect, it should be remembered that bite strength is not everything; despite its large size, the Great White Shark (Carcharadon carcharias) has a relatively weak bite, but it makes up for it with heavily serrated teeth, force of impact when attacking prey, and side-to-side head shaking to saw through its food. Crocodilians, by contrast, have very strong bite forces but they don’t saw through prey or chew, the emphasis being holding on to struggling prey and drowning it before ripping it apart. Such considerations bring us to another point mentioned above in our discussion of scimitar-tooths vs. dirk tooths in that the famous dirk-toothed cats like Smilodon were more powerfully built, seemingly focusing on bringing a large animal down to the ground and then delivering devastating bites once the stomach and neck were exposed (a process that would be made easier by groups working together, as seen in modern examples like lions bringing down giraffes or elephants).
A group of lions brings down a giraffe.
A group of lions brings down an elephant.
A new paper, just out in PNAS, does take the powerful neck muscles of Smilodon into account, however, and the information from the new models appear to corraborate the modern understanding of a felid that captured and killed prey in a way quite different from Panthera. From McHenry, et al.;
Our results demonstrate that bite force driven by jaw muscles was relatively weak in S. fatalis, one-third that of a lion (Panthera leo) of comparable size, and its skull was poorly optimized to resist the extrinsic loadings generated by struggling prey. Its skull is better optimized for bites on restrained prey where the bite is augmented by force from the cervical musculature. We conclude that prey were brought to ground and restrained before a killing bite, driven in large part by powerful cervical musculature. Because large prey is easier to restrain if its head is secured, the killing bite was most likely directed to the neck. We suggest that the more powerful jaw muscles of P. leo may be required for extended, asphyxiating bites and that the relatively low bite forces in S. fatalis might reflect its ability to kill large prey more quickly, avoiding the need for prolonged bites.
Hunting isn’t the only aspect of sabercat predation that seems to have differed from modern carnivores; they way they ate (and what they ate) is somewhat at variance with modern forms, as well. As is apparent at this point, the contact of the canines with bones would have been avoided, and it seems that the hard parts of the skeleton would have been avoided when a sabercat was consuming it. This could differ among different groups (perhaps some of the shorter-toothed forms not being so finicky about bone), but research into microwear patterns on teeth of Smilodon don’t seem to match with wear patterns of any living carnivores, suggesting a different dietary preference. It could be hypothesized, then, that creatures like Smilodon primarily consumed the soft parts of the carcass or what could be removed without too much damage to the teeth, and it should be remembered that living big cats often do not eat every part of the skeleton. Some, like cougars, have favored parts that they eat but end up leaving as much as 40% of the carcass behind. Other predators, especially bone-crushing ones, could take advantage of the leftovers, although the felids might have had to eat quickly as some of their osteophagus competitors may not have been patient (and, in fact, lions and hyenas often fight over kills and steal them from each other today).
Given all the prior considerations, it now seems that sabercats specialized in bringing down relatively large prey down quickly (some likely working in groups to do so), killing the victims by slashing open their stomachs or slicing through the blood vessels of the neck. This would be a much messier, but quicker, method than employed by living big cats, although the limitation of food sources likely caused in the eventual downfall of sabercats. Hypercarnivory can be a dangerous adaptive path to go down, and cats are clearly the most meat-dependant of the Carnivora, but it seems that extinct forms took their dental and dietary specialization above and beyond what is seen today. The price paid for such adaptations ended up being extinction, but given how many times they have shown up in the history of life on this planet, someday there may again be a saber-toothed predator stalking the shadows.
References;
Anyonge, W. “Microwear on Canines and Killing Behavior in Large Carnivores: Saber Function in Smilodon fatalis” Journal of Mammalogy, Vol. 77, No. 4 (Nov., 1996), pp. 1059-1067
Christiansen, P. “Canine morphology in the larger Felidae: implications for feeding ecology.” Biological Journal of the Linnean Society. Vol. 91, No. 4 (Aug., 2007), pp. 573-592
Christiansen, P. “Sabertooth characters in the clouded leopard (Neofelis nebulosa Griffiths 1821).” Journal of Morphology, Vol. 267, No. 10 (Jul., 2006), pp. 1186 – 1198
Christiansen, P. and Wroe, S. “Bite Forces and Evolutionary Adaptations to Feeding Ecology in Carnivores.” Ecology, Vol. 88, No. 2 (Feb., 2007), pp. 347–358
Cope, E.D. “The Amblypoda (Continued).” The American Naturalist, Vol. 19, No. 1. (Jan., 1885), pp. 40-55.
Cope, E.D. “The Tertiary Marsupialia.” The American Naturalist, Vol. 18, No. 7. (Jul., 1884), pp. 686-697.
Emerson, S.B., and Radinsky, L. “Functional Analysis of Sabertooth Cranial Morphology.” Paleobiology, Vol. 6, No. 3. (Summer, 1980), pp. 295-312.
Leutenegger, W., and Kelly, J.T. “Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates.” Primates, Vol. 18, No. 1 (Jan., 1977), pp. 117-136
Lucas, P.W., Corlett, R.T., and Luke, D.A. “Sexual dimorphism of tooth size in anthropoids.” Human Evolution Vol. 1, No. 1 (Feb., 1986), pp. 23-39
Marsh, O.C. “The Fossil Mammals of the Order Dinocerata.” The American Naturalist, Vol. 7, No. 3. (Mar., 1873), pp. 146-153
Martin, L.D., Babiarz, J.P., Naples, V.L., and Hearst, J. “Three Ways To Be a Saber-Toothed Cat.” Naturwissenschaften, Vol. 87, No. 1 (Jan. 2000), pp. 41-44
McHenry, C.R., et al. “Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation.” PNAS, Published online before print October 2, 2007
Salesa, M.J., et al. “Aspects of the functional morphology in the cranial and cervical skeleton of the sabre-toothed cat Paramachairodus ogygia (Kaup, 1832) (Felidae, Machairodontinae) from the Late Miocene of Spain: implications for the origins of the machairodont killing bite.” Zoological Journal of the Linnean Society, Vol. 144, No. 3, (Jul., 2005) pp. 363-377
Salesa, M.J., et al. “Inferred behaviour and ecology of the primitive sabre-toothed cat Paramachairodus ogygia (Felidae, Machairodontinae) from the Late Miocene of Spain” Journal of Zoology, Vol. 268, No. 3 (Mar., 2006), pp. 243-254
Simpson, G.G. “The Function of Saber-Like Canines in Carnivorous Mammals.” American Museum Novitiates, August 4, 1941
Therrian, F. “Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators.” Journal of Zoology, Vol. 276, No. 3 (Nov., 2005), pp. 249-270
Therrian, F. “Feeding behaviour and bite force of sabretoothed predators.” Zoological Journal of the Linnean Society, Vol. 145, No. 3 (Nov., 2005), pp. 393-426
Van Valkenburgh, B., and Molnar, R.E. “Dinosaurian and mammalian predators compared.” Paleobiology, Vol. 28, No. 4 (Dec., 2002), pp. 527–543
Walker, Alan. “Mechanisms of honing in the male baboon canine.” American Journal of Physical Anthropology, Vol. 65, No. 1 (?, 1984), pp. 47 – 60
Wroe, S., McHenry, C., and Thomason, Jeffery. “Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa.” Proceedings of the Royal Society B, Vol. 272, No. 1563 (Mar., 2005), pp. 619-625
When chimpanzees (Pan troglodytes) appear in documentaries they are often shown inhabiting relatively dense tropical forest, their lives taking place within the green refuge of the forests. As with any other species that is spread over a considerable distance, however, different populations of chimpanzees have different habits, and one of the most remarkable populations are those around Mt. Assirik. Located in the southeastern part of the Parc National du Niokolo-Koba in Senegal, the chimpanzees in this area have to deal with a local ecology that is drier and more open than some of their relatives elsewhere, and their behavioral adaptations to the environment is of great interest to those study human origins.
The Mt. Assirik study area is remarkable in that 55% of the habitat is open grassland, only about 37% being woodland of varying density and only 3% being more dense forest (the remaining area being made up of bamboo forest and isolated trees). Such open spaces allow some of the major Carnivora of Africa to live in close proximity to the chimpanzees; Lions (Panthera leo), Leopards (Panthera pardus), Wild Dogs (Lycaon pictus), and Spotted Hyenas (Crocuta crocuta) are all frequently seen in the area. As if having so many predators at their doorstep were not enough, the Mt. Assirik area seems to have fluctuations of food that aren’t correlated with seasonal changes, and in the dry season water is the most prized of any resource. The apes are not entirely helpless in the face of such pressures, however, and they’ve been behaviorally adapted in some very interesting ways.
Given a choice, the Mt. Assirik chimpanzees prefer to spend their time in the denser areas of forest, but shifting food resources sometimes require them to move across large expanses of open grassland in order to find nourishment. Wandering out onto the open plains alone is so dangerous as to nearly be suicidal, and the apes form large mixed groups when they have to move across the plains. During this time they are at their most vulnerable, especially since they would be unlikely to outrun any of the major predators (especially those that hunt in packs), and they are extremely alert when undertaking such a journey. What is perhaps most striking of all, hearkening back to Raymond Dart’s “Savanna Hypothesis,” is the fact that the chimpanzees sometimes stand up to get a better look at their surroundings, potentially spotting predators before they get too close, although such an observation should not be taken as a sweeping vindication of Dart’s ideas of human evolution.
The presence of just one tree or a few trees spaced far apart doesn’t help the chimpanzees much either; mothers with children and individuals spent much less time in the sparser woodland areas than in the forest, mixed groups seemingly having to issues with the woodlands. Why should this be so? Well, leopards can climb trees (and often do so to stash their kills), as well as lions, and so simply climbing a tree does not equal escape. Lone chimpanzees are far more comfortable in a habitat where they can climb a tree and move through the canopy out of reach of their assailants, something that is not possible in woodlands. The predators may also have another effect on the diet of the chimpanzees; the Mt. Assirik chimps do not seem to eat young ungulates or monkeys, although such behaviors have been made famous where it has been observed (i.e. Gombe). This may be due to some competition, but it may also be due to the restricted forested habitat and the fact that chimpanzees would have to enter the habitat of the carnivores in order to capture young ungulates, predators being likely to quickly learn about any kills that had been made.
Indeed, the Mt. Assirik population is remarkable in that it often moves long distances in order to obtain food as it becomes available, relying on numbers and vigilance to protect itself from predators when it’s habitat only offers a few isolated islands of relief. Although humans did not evolve from modern chimpanzees, this population may give researchers some idea of the behavior patterns of our ancestors when faced with similar constraints when forests became sparser and the plains were filled with predators. Such social behavior is not the only thing that makes the Mt. Assirik chimpanzees stand out, however; they also make use of Baobab trees in a very interesting way.
By now many people are familiar with the ability of chimpanzees to use a piece of wood as a hammer to break a nut placed upon an “anvil” of rock or tree root; such footage has been shown in television programs again and again. Such behavior did not come out of nowhere, however, and the way Mt. Assirik chimpanzees open nuts may represent a stage of tool use that precedes the hammer-and-anvil technology. While it had been disputed for some time whether the Mt. Assirik population used hammers and anvils or just anvils, recent studies have shown that they are cracking open the hard nuts of the tree on branches and not using a hammer. While we might think of an “anvil” as something that can only be used in conjunction with a hammer, mechanically this isn’t necessarily so, and the Mt. Assirik chimpanzees bang the hard nuts they collect on the branches of the tree (therefore staying aloft, not coming down to use stones or the roots of the tree), the tree itself being the anvil.
Given the basal usage of anvils by the Mt. Assirik chimps and the use of hammers and anvils elsewhere, it becomes possible to hypothesize about the evolution of stone tool use in our own ancestors. The starting point was likely similar to what is exhibited by the Mt. Assirik chimpanzees, banging hard nuts on trees or rocks in order to open them (thus preventing damage to the teeth, if it even would be possible to open the nuts using their jaws). The next step would be adding a hammer, possibly wooden (as seen in some groups today) or possibly stone. At this stage any combination of wood or stone hammers and anvils could be used, but tool use would probably not progress until a population was using stone hammers and stone anvils to open foods. In such a scenario, the apes would sometimes miss their targets and flake off bits of stone, an accident that would shape the tools. When a certain cognitive leap was made, the apes could then move from accidentally flaking their tools to doing it intentionally to truly be making tools rather than making use of naturally occurring bits of wood and stone. The reality of the situation may be forever lost to us, ancient tool use before the knapping of stone became prevalent being notoriously hard to discern, but such a line of behavioral descent is not unreasonable and seems to allow further development merely by chance combinations of naturally occurring resources.
Such a discussion is only a brief sketch based upon what I have only recently learned myself, but I hope that it has been at least somewhat informative. Different populations of chimpanzees show different behaviors and live in differing ecologies, and it would be a mistake to assume what the famous Gombe chimpanzees are doing holds true for all the other populations. Another population that I soon intend to write about spends time in caves, probes trees for bush babies, and may even have the beginnings of a fire culture; others do not show the same exact behaviors, but they have their own cultures and reactions to the local ecology. While we should be careful in analyzing the living populations of chimpanzees and their perceived similarities to humans, it would be foolish to think that they can tell us nothing of our own past, and if very well may be that some of traits (behavioral or otherwise) they now exhibit were present in our own lineage, vignettes of evolutionary history being replayed with different actors in our own time.
What is it about some people in Texas wanting to slaughter animals for no good reason? According to news reports out today, a number of bats (an actual estimate of the “infestation” not being given) found their way into Lanier Hall East at Texas Southern University, and a number of the young men in residence thought to make a sport out of the lost (and probably confused) bats. [Note: The following video contains offensive language];
While many of the news reports expressed worries about rabies (although no one seems to have been bitten), the people who killed any number of the flying mammals apparently received no rebuke for their cruelty to the animals. They were not being attacked or molested by the bats, and if there was a problem why not leave? Swinging away at the creatures, probably disoriented and frightened, reveling in the smack of the furry bodies on the linoleum, is far from mature, responsible, or even intelligent. Bats are absolutely amazing animals, and despite the rather pungent smell of guano, I was lucky enough to observe Little Brown Bats leaving their roost over a number of nights last summer at Stokes State Forest. There’s really no excuse for what I can only term cruelty on the part of the students in the videos.
Some Texans take a more healthy interest in bats, however, (although their reactions to a bat in nature and a bat in the house might be different depending on who we’re talking about), and plenty of people show up to watch bats emerge from beneath the Congress Ave. bridge in Austin, TX;
Bat really are amazing mammals, and I am remiss in not writing about them more often (and so they’ve been duly added to my ever-growing list of things to learn/write about). In the meantime, enjoy this clip from The Life of Mammals featuring a particular bat that has a relatively unusual way (as far as bats go) in detecting prey.
Note: Welcome, visitors from The Sandwalk and Pharyngula! I am certainly humbled by the amount of praise and attention this post has received, and although it’s not as scientifically rigorous as I would have liked to be (I still have much to learn), I hope that you find it to be an enjoyable read all the same.
Update: I’ve created something of an appendix to this article about how creationists have presented horse evolution in some of their books. It can be found here.

One of Charles R. Knight’s interpretations of Eohippus
When the name of O.C. Marsh is invoked, it is often to tell of his participation in the great “Bone Wars” of the late 19th century, sparring with fellow osteophile E.D. Cope in the pages of the New York Herald. Twisted tales of deceit and sabotage were promulgated in the sensationalist paper, and while both men helped to bring about an American revolution in vertebrate paleontology, the scars of their bitter squabbling have yet to fully heal. Such scientific in-fighting might seem worthy only of a historical footnote or an introduction to the stereotyped image of “smash-and-grab” paleontology of the time which is almost romantically referred to, but the truth of the matter goes far deeper than the public beard-pulling that is so often remembered.
The tiff between Cope and Marsh is strange in that is seems to exist in the popular literature out of time, removed from the context in which it had originally existed. Charles Darwin had published his earth-shaking work On the Origin of Species by Natural Selection a scant 31 years before the ink almost ran red with rage on the pages of the Herald, the question of evolution being of far more importance in the public consciousness than dinosaurs. The full establishment of the dinosaur as a cultural (and dare I say, mythical) creature in the mind of the American public only seemed to take place after the Bone Wars, the appointment of Henry Fairfield Osborn to the American Museum of Natural History (specifically hired to establish a vertebrate paleontology program) and the popular reports of the dinosaur that carried the namesake of Andrew Carnegie, Diplodocus carnegei, being the more immediate beginnings of the public’s love affair with the extinct creatures. Before Brontosaurus and Tyrannosaurus became household names, the public eye was focused upon horses and birds.
The latter half of the 19th century was a stirring time for biological science, especially involving the new areas of vertebrate paleontology and evolution, the august authorities in England keeping on eye on the up-and-comers starting their own careers in the states. Early on, paleontologist E.D. Cope impressed T.H. Huxley with his 1866 discovery of Laelaps aquilunguis, but in a paleontological clean-sweep Marsh would eventually have his name attached to Cope’s dinosaur and the admiration of not only T.H. Huxley, but Charles Darwin himself. As for the renaming of Laelaps, Marsh found that the name was already taken by a genus of mite, renaming the New Jersey greensand dinosaur Dryptosaurus in 1877 (although Cope, throughout the rest of his career, called the dinosaur Laelaps). It would take more than some taxonomic shuffling to impress the eminent British anatomists and paleontologists, however, and Marsh’s ticket into Huxley’s good graces came in the form of toothed Cretaceous birds like Hesperornis (Marsh, 1872).
While the discovery of ancient bones was exciting to some, evolution was an even more popular topic, and the question that surrounded every fossil was “How does this fit into the grand scheme of evolution?” The 1861 discovery of Archaeopteryx from the lagerstatten of Solnhofen, Germany seemed to arrive right on cue to confirm that evolution had taken place in times previously referred to as “antediluvial”, and Marsh’s subsequent discovery of birds with teeth in the American West further confirmed the notion that aves had evolved from reptilian ancestors (Huxley even being so progressive as to name the dinosaurs as the probable ancestral stock). Charles Darwin himself recognized the importance of Marsh’s discoveries as well, and two years after Marsh visited Darwin at Down House in 1878, Darwin wrote the following letter to Marsh on or about August 31, 1880;
I received some time ago your very kind note of July 28th, & yesterday the magnificent volume. I have looked with renewed admiration at the plates, & will soon read the text. Your work on these old birds & on the many fossil animals of N. America has afforded the best support to the theory of evolution, which has appeared within the last 20 years. The general appearance of the copy which you have sent me is worthy of its contents, and I can say nothing stronger than this.
With cordial thanks, believe me yours very sincerely,
Charles Darwin
Toothed birds were not Marsh’s only claim to evolutionary fame, however; by 1876 his assistants had collected enough fossil horse material to show that the horse was not “a gift from the Old World to the New” (as an European authority had once said during a lecture), but quite the reverse. In fact, the timing of the discovery and study of the horses could not have been better for Marsh, as in 1876 T.H. Huxley visited Yale and was duly impressed with the American Professor and his fossil horses. Huxley was absolutely enthralled by Marsh’s fossil equids, and Huxley’s son Leonard wrote of the visitation upon the New World horses as follows;
At each inquiry, whether he had a specimen to illustrate such and such a point or exemplify a transition from earlier and less specialized forms to later and more specialized ones, Professor Marsh would simply turn to his assistant and bid him fetch box number so and so, until Huxley turned upon him and said ‘I believe you are a magician; whatever I want, you just conjure up.'”
Restoration of Eohippus. From “The Dawn Horse or Eohippus” by Chester Stock (1947).
Huxley even featured Marsh’s discoveries of toothed birds and fossil horses in a set of three lectures he delivered at Chickering Hall in New York, the visit of such a famous evolutionist being front page news (with Marsh sharing in good press since his fossils were discussed by so prominent a figure as Huxley). The only thing that could have made the event sweeter would have been the knowledge of an ancestral horse with five toes (what was regarded as the “primitive” condition for mammals as far as digits go), and Huxley prophesied that such a creature would likely be found in North America. In truth, while it did not precisely fit the bill, a horse bearing a vestigial fifth toe had already been found and was collecting dust in Marsh’s Peabody museum. Writing to Huxley on July 12, 1877, Marsh revealed that little Eohippus (a name that was given up when it was discovered that Richard Owen’s Hyracotherium had priority, only to be later changed back to Eohippus in recent years) had been right under his nose all along;
I had him “corralled” in the basement of our Museum when you were there, but he was so covered with Eocene mud, I did not know him from Orohippus. I promise you his grandfather in time for your next horse lecture if you will give me proper notice.
Although the popular press did not take much note of the re-discovery of Eohippus, Huxley was well pleased, and promised to show Marsh all the “lions” of British science during his aforementioned 1878 visit. Such close ties would be important to Marsh later on, serving to keep Cope out of some respectable circles as well as giving Marsh a good amount of prestige. Oddly enough, however, Cope had his own horse genealogy (although ignoring Marsh’s labels) that went from four toes to one, and it was Cope’s “dawn horse” that provided the basis for some of the first Eohippus reconstructions, not Marsh’s. While Cope missed out on a golden opportunity in 1872 when he was provided a jaw fragment of an early horse, Marsh’s skeleton (as far as I can tell) remained locked away while Cope’s employee J.L. Wortmann uncovered the rest of Cope’s specimen of Eohippus in 1880. Cope named his animal Protorohippus, and it was his reconstruction that ultimately influenced Charles R. Knight and, subsequently, Rudolph Zallinger when he painted his famous Age of Mammals mural. For those who did not get at least a chuckle out of the last line, Zallinger created his mammalian masterpiece for the Peabody museum, the very establishment that O.C. Marsh had created to start his professional career.
As can be said of any scientist, however, Cope and Marsh were both products of their time and (especially in their respective cases) their egos, and while the fact that horses evolved was proved beyond doubt, both men made mistakes when it came to evolution. While Cope, late in his career, bemoaned the fact that Marsh had poisoned the well when it came to making connections with Huxley and other British scientists, it is doubtful that Cope would have lasted long amongst those of the Darwinian school of evolution. In the 1896 book The Primary Factors of Organic Evolution, Cope saw the evolution of the horse being orthogenic, or proceeding in such a way as to imply direction towards a more adapted or perfected form. As this concerns horses, Cope wrote;
Examination of all these genealogical lines reveals a certain definiteness of end and directness of approach. We discover no accessions of characters which are afterwards lost, as would naturally occur as a result of undirected variation. Nor do we discover anything like the appearance of sports along the line, the word sport being used in the sense of a variation widely divergent from its immediate ancestor. On the contrary, the more thorough becomes our knowledge of the series, the more evident does it become that progressive evolution has advanced by minute increments along a definite line, and that variations off this line have not exerted an appreciable influence on the result.
Such notions would have gotten Cope banned from Finch’s Beak gentleman’s association (if one had actually existed), the concept of directed evolution undermining one of the most important points that Darwin had attempted to make about the “transmutation” of life on earth. As we shall later see, however, such notions of orthogenesis may have had some influence on one of Cope’s latter-day pupils, Henry Fairfield Osborn, as well.

An illustration of the horse “Clique,” which had an extra toe on each fore-foot. Marsh examined this horse while still alive, and the horse was donated to Yale after its death in 1891. From Marsh, O.C. 1892. “Recent Polydactyl Horses.”
Marsh, as has already been determined, definitely had the attention of the progenitors of evolution by natural selection, and through the efforts of Matt Wedel, Randy Irmis, and Mike Taylor a number of Marsh’s writings have become available for viewing on the internet (The Marsh Repository). In a 1879 paper published some time after Huxley’s visit, “Polydactyle Horses, Recent and Extinct,” Marsh prefaces the rather short fossil section with several pages about known horses within recent history that had extra digits. The most typical condition for the differing equines was having an extra digit on the inside of the front hooves, one that did not touch the ground. Coupled with a brief appeal to similar observations of extra toes from development, this approach was indeed a wise one; not only do most living horses have vestiges of digits that have been lost, sometimes a multi-toed condition still occurs in living animals, seemingly fitting with the same trends seen in extinct genera.

O.C. Marsh’s concept of “The Geneology of the Horse,” a decidedly straight-line progression. From Marsh, O.C. 1879. “Polydactyly Horses, Recent and Extinct.”
What is notable about Marsh’s interpretation of the history of horse evolution is how straightforward it is. Although missing Cope’s differing evolutionary hypotheses, Marsh makes no qualifications about the fossils he found representing only the “types” of different horses; horses evolved along a straight line, and while a few steps may be missing, it was not indicative of the widely branching pattern recognized by later scientists. The “extraneous” toes seem to become reduced in a gradual fashion, while size and tooth height increased (although the patterns on the teeth, as can be seen in the illustration, vary quite a bit in the “higher” forms). Perhaps Marsh’s adherence to a strict linear progression was at least partly inspired by the diagnosis of Huxley. In an obituary written by Marsh to commemorate Huxley’s life, Marsh made special mention of his horses;
One of Huxley’s lectures in New York was to he on the genealogy of the horse, a subject which he had already written about, based entirely upon European specimens. My own explorations had led me to conclusions quite different from his, and my specimens seemed to me to prove conclusively that the horse originated in the New World and not in the Old, and that its genealogy must be worked out here. With some hesitation, I laid the whole matter frankly before Huxley, and he spent nearly two days going over my specimens with me, and testing each point I made. He then informed me that all this was new to him, and that my facts demonstrated the evolution of the horse beyond question, and for the first time indicated the direct line of descent of an existing animal.
Such interpretations of evolution and the fossil record could only exist within a certain paleontological framework; the more bones that were found from different times and locales the more the old notions would splinter and crack. Vertebrate paleontologists who would succeed Cope and Marsh could not study what they did not have, however, but they still recognized the importance of the horse in showing evolution to be a reality. In 1891 Henry Fairfield Osborn, an independently wealthy Princeton professor and one of E.D. Cope’s friends and supporters during the embroiled Herald fiasco, was appointed the first curator of vertebrate paleontology at the American Museum of Natural History in New York City. The museum was somewhat embarrassed at not possessing any sizable collection of vertebrate fossil material, and even though Cope eventually sold some of his collection to the AMNH for a sum that disappointed the beleaguered Philadelphian, the halls of the great institution were still found wanting of ancient creatures that would bring it notoriety.
Osborn, despite his off-kilter ideas about human evolution that plagued his later years, largely made the AMNH what it is today, having some of the best and brightest collectors and preparators of the 20th century under his employ. While such gems as Barnum Brown’s two Tyrannosaurus rex skeletons, the specimen that remains on display today being Brown’s self-confessed “favorite child,” definitely helped to make the museum famous, some of Osborn’s favorite subjects were the fossil horses. Early on in his career, Osborn attempted to raise $10,000 from museum trustees for a project involving horse evolution, but the appeal was denied. Osborn kept at it and eventually succeeded, however, securing $15,000 from William C. Whitney in 1897, funds used to send collectors and curators like James W. Gidley, Bill Thomson, W.D. Matthew, and Walter Granger out into the field to collect ever more horses from Texas, South Dakota, Colorado, and other locales. Indeed, Osborn soon had many new horse fossils to study and display, creating one of the most notable (and among Biblical fundamentalists, controversial) displays of evolution ever presented to the public.
*(WWII caused the museum to send the first, more incomplete Tyrannosaurus rex skeleton, to the Carnegie Museum out of fear that the museum would be bombed and both would be lost. This may seem like an ill-founded fear, but many fossils like Spinosaurus were lost because German museums were struck with Allied payloads.)
Osborn did much to enhance the AMNH collections during the close of the 19th century, although his rather strange views about mammalian evolution (fueled in part by racism and part by Osborn’s membership in the Presbyterian church) never found wide acceptance. Despite his pet hypotheses, Osborn sent paleontologists far and wide in search of specimens to confirm his ideas, and at least in the case of the Roy Chapman Andrews expeditions during the early 1920’s, unexpected boons abounded. While Marsh held that he had moved horse ancestry out of the Old World and safely into America’s domain, Osborn saw the origin of major placental mammalian groups stemming from Asia (including the origin of humans), the hypothetical five-toed ancestor of the horse remaining elusive in North America because it was “really” buried somewhere in Asia. Osborn described his hypothesis as follows;
In the dispersal center, during the Age of Reptiles and the beginning of the Age of Mammals, there evolved the most remote ancestors of all the higher kinds of mammalian life which exist today, including, for example, the five-toed horses, which have not yet been discovered in either Europe or America. That the very earliest horses known in either Europe or America were four-toed indicates that their ancestors may have lost their fifth toe while still resident in the Asiatic homeland.
Roy Chapman Andrews did not bring Osborn any Asiatic five-toed horses from the expeditions into Mongolia in the early 1920’s, although the mammals Paraceratherium and Andrewsarchus were exciting enough in and of themselves. .
The lack of the most ancestral mammalian fossils did not stop Obsorn from attempting to further his own hypotheses, however, and in order to understand how straight-line evolution may have been presented at the AMNH we need to know how Osborn obfuscated the role of “chance” in evolution (using it almost in the same context as modern creationists do), calling the idea that natural selection works on random variations a “dogma.” Osborn instead preferred an Aristotelian “law,” quoting the philosopher in his 1917 book The Origin and Evolution of Life;
So far as law is concerned, we observe that the evolution of life forms is like that of the stars: their origin and evolution as revealed through palaeontology go to prove that Aristotle was essentially right when he said that “Nature produces those things which, being continually moved by a certain principle contained in themselves, arrive at a certain end.”
Such a notion could be regarded as the “Restless Gene” hypothesis, with what Osborn then referred to as the “hereditary-chromatin” in the animal filling needs as they arose in order to achieve a particular end. Despite his confusion about the role of “law” and “chance” in nature, Osborn did recognize that there were certain ratios in limb structures that were present in animals filling different ecological niches, even closely related ones. In the same book, Osborn writes the following about early horses;
No form of sudden change of character (saltation, mutation of de Vries) or of the chance theory of evolution accounts for such precise steps in mechanical adjustment [as in the limb structure of horses]; because for all the proportional changes, which make up ninety-five percent of mammalian evolution, we must seek a similar cause, namely, the cause of acceleration, balance or persistence, and retardation. This cause may prove to be in the nature of physiochemical interactions regulated by selection. The great importance of selection in the evolution of proportion is demonstrated by the universal law that the limb proportions of mammals are closely adjusted to provide for escape from enemies at each stage in development.
This chain of reasoning, such as it is, nearly works backwards from evolution’s “products” (which it is never done fiddling with), much like the lampoon (which I believe stems from Voltaire, although I have been unable to find the quote) that the nose was placed on the human face to hold up ones glasses.

Assemblage of bones, illustrated as discovery in situ, of the Pleistocene horse Equus scotti. From Gidley, James Williams. 1900 “A new species of Pleistocene horse from the Staked Plains of Texas“. Bulletin of the AMNH ; v. 13, article 13.

A mounted skeleton of Equus scotti at the AMNH, constructed out of two skeletons. From Gidley, James Williams. 1901. “Tooth characters and revision of the North American species of the genus Equus.” Bulletin of the AMNH ; v. 14, article 9.
Even though Osborn’s ideas of evolution did not catch on, the idea of horse evolution as a more-or-less straight line was still a popular one, at least in works and representations meant for public consumption. The diversity of fossil horses, thanks to many of the expeditions undertaken by Osborn’s department at the beginning of the 20th century, had considerably expanded, and the idea of an evolutionary “bush” for horses began to take root. Such a representation can be seen in one such generalized and “primitive” bush provided by J.W. Gidley in a 1907 paper on horses from the Miocene and Pliocene of North America. Indeed, the diversity of three-toed forms suggested that ancestry was perhaps more complicated than previously thought, more than one form of horse existing at any one time depending on the available habitats. Osborn noted this in his 1917 book as well, but it seemed to be only a supplementary bit of information behind his ideas of a biogenetic law. One of Osborn’s hires, J.W. Gidley, had a more accurate view of horse evolution, however, and produced the first known branching phylogeny of horses through the Miocene and Pliocene.

A hypothesis as to the relationships of horse subfamilies by J.W. Gidley in 1907. This is the first known branching diagram for horse evolution. From Gidley, James Williams. 1907. “Revision of the Miocene and Pliocene Equidae of North America.” Bulletin of the AMNH ; v. 23, article 35.
As can be seen from Marsh’s earlier phyletic progression, much of horse evolution seemed to be dictated by features of teeth, the number of toes, and certain aspects of the skull, but as Gidley notes in his paper more material is needed if we are to truly understand the relationships of horses. Indeed, things were not so clean and neat as implied by Marsh’s illustration, even with the inclusion of new taxa. In the summary of the research, Gidley concludes;
As at present understood, the fact seems to be fairly well established that there is a considerable phyletic hiatus between the groups of the Equidae as above subdivided, which are as yet not bridged over by intermediate forms. Such a hiatus seems especially marked between the Anchitheriinae and the Protohippinae, while these groups greatly overlap each other in time. So far as indicated by any known species the Anchitheriinae could not well have stood in direct ancestral line to the latter group or to the Equiinae. There seems also to be almost as decided a gap between the Anchitheriinae and the known species of the older group, the Hyracotheriinie. The Equiinae may well have been derived from some species of the Protohippus division of the Protohippinae.
Outside of engaging in a more detailed study, Gidley also made note that various genera of horses overlapped in time with each other. While this does not rule out anagenesis entirely, it is a problem if there is such a large diversity of horses with similar features living alongside each other rather than a few isolated populations moving in a straight-line progression. The overlap was recognized and illustrated by W.D. Matthew almost 20 years after Gidley’s paper, showing where and when fossil horses existed;

Visual representation of the geological span and geographical ranges of equids through the Cenozoic. Such a representation could easily be misunderstood as endorsing straight-line evolution of horses. From Matthew, W.D. 1926. “The Evolution of the Horse: A Record and Its Interpretation” The Quarterly Review of Biology, Vol. 1, No. 2., pp. 139-185.
While the illustration, if followed closely, does show a branching pattern of evolution, to an untrained eye the evolution of horses through time seems to go in a relatively straight line, the overlap seemingly giving way to an almost orthogenic trend. I doubt that Matthew’s article was regular Sunday night reading for families of the late 1920’s and so I doubt that it contributed directly to mistaken notions of horse evolution, but another illustration from the same paper could more easily cause confusion;

A simplified, “straight-line” version of horse evolution (Click the image for a larger version). This figure was also reproduced in George McCready Price’s The Predicament of Evolution. From Matthew, W.D. 1926. “The Evolution of the Horse: A Record and Its Interpretation” The Quarterly Review of Biology, Vol. 1, No. 2., pp. 139-185.
This model is similar to Marsh’s (see above) in that horses seem to have followed a very simple ancestor/descendant progression through time. While it is true that living horses did have ancestors with multiple toes and we could trace their line backwards through time to the exclusion of other closely related genera, diagrams like this one seem to have “won out” in the public mind over those that more fully encompassed horse diversity. A 1940 paper by R.A. Stirton would be much clearer when it came to the branching horse lineage;

From Stirton, R. A. 1940. Phylogeny of North American Equidae. Bull. Dept. Geol. Sci., Univ. California 25(4): 165-198.
Stirton’s illustration is interesting as it shows a fairly straightforward line of descent through Miohippus is the Upper Oligocene, Miohippus giving rise to some side branches that would eventually go extinct before modern times. The radiation of the ancestors and close relatives of modern horses did not start, according to the phylogeny, until the Upper Miocene and Merychippus, Pliohippus eventually giving rise to Equus in the Upper Pliocene. Further, it is interesting to see how close Stirton’s phylogeny is to the work of later researchers, especially that fossil horse authority Bruce McFadden;

From MacFadden, Bruce. 1985. “Patterns of Phylogeny and Rates of Evolution in Fossil Horses: Hipparions from the Miocene and Pliocene of North America” Paleobiology, Vol. 11, No. 3. (Summer, 1985), pp. 245-257.
The phylogeny is extremely similar to Stirton’s through Parahippus, but the upper branches are a bit more detailed. Instead of having the genus Equus be a descendant of Pliohippus, Pliohippus is relegated to an offshoot that goes extinct before the Pliocene, the genus Dinohippus giving rise to Equus and the recent horses of the New and Old World in that genera. We will come back to the work of MacFadden later, but it is important to note how close the ideas of researchers in decades past were with modern understanding in this area.

From Quinn, J. H. 1955. Miocene Equidae of the Texas Gulf Coastal Plain. Bur. Econ. Geol., Univ. Texas Pub. 5516: 102 pp. (Click for larger image)
J.H. Quinn’s 1955 phylogeny of the horses of the Texas Gulf Coastal Plain was even more wildly branching than Stirton’s, and while Quinn’s focus was a bit more narrow, the tree is much more divergent. Other researchers had the genus Equus arising in the late Pliocene (and even as late as the Pleistocene), Quinn’s version has Equus appearing as early as the middle Miocene, Merychippus, again being nominated as the progenitor of all the subsequent forms in the area. While this version of horse evolution has been extensively reshuffled and revised, it is important to note that the idea that horses evolved in a straight line was well out of fashion by the middle of the 20th century at the very latest. Why, then, did it hang on for so long in the public mind?

A mount of Mercyhippus isonesus quintus. From Simpson, George Gaylord. 1932. “Mounted skeletons of Eohippus, Merychippus, and Hesperosiren.” American Museum novitates ; no. 587
Part of the problem with museums is that it takes a lot of time, money, and effort to revise exhibitions, and for some time the American Museum of Natural History horse display (THE display that illustrated horse evolution for many years) followed a progression like that of W.D. Matthew’s simplified diagram (see above). While this was eventually changed when the fossil halls were refurbished, it still seemed to show a straight line of descent, and even the display that stands on the fourth floor of the museum today reflects such a transition. If you read the plaques and take the time to compare the skeletons the branching nature of horse evolution is apparent, but the fossils themselves are arranged from Eohippus to Equus in a two parallel straight lines, showing an overall smaller-to-larger and many-to-one toe progression. Likewise, popular books on evolution and paleontology seemed hard-pressed to let go of straight-line evidence. While it could be said that such books were correct in that we could follow the line of descent from modern horses backwards to the exclusion of other groups, this approach seems to do more harm than good in the long run. Take A.S. Romer’s Man and the Vertebrates: Vol. I, for example. Originally published in 1933, my 1954 Pelican Books paperback edition shows the fossil limbs of Eohippus, Miohippus, Merychippus, and Equus from left to right, once again giving the illusion of a pure line of anagenesis. No hint of a larger diversity is given outside a brief mention of the modern forms of Zebra, Ass, and Prezwalski’s Horse.

Comparison of Eohippus to Equus. There’s a lot of evolution in that dashed line. From “The Dawn Horse or Eohippus” by Chester Stock (1947).
The 1966 edition of Romer’s Vertebrate Paleontology fairs better overall, but is still found wanting. The same straight-line illustration I just mentioned is found in the section treating perissodactyls as a group, and the skeletons of Eohippus, Mesohippus, and Hippidion are shown left to right across pages 266 and 267. While the text does mention an overall diversity of forms, as well as using certain genera for the “type” from which modern horses evolved, the overall visual impression of simple anagenesis remains. Again, I doubt the casual reader picked up Romer’s book for light nightly reading, but it is strange that the progressive ideas about evolution during that time are so poorly represented.
A similar time-capsule is Edwin Colbert’s Evolution of the Vertebrates, originally printed in 1955. The 1966 edition is the one that I acquired, and it is an interesting contrast to Romer’s book. At first Colbert seems to fall into the same trappings of straight-line evolution, showing a simple progression (in text with arrows) from Hyracotherium (Eohippus) -> Orohippus ->Epihippus -> Mesohippus -> Miohippus, spanning the Lower Eocene to the Upper Oligocene. After this progression, however, Colbert does note that there was a proliferation in forms;
By the end of the Oligocene epoch the horses had through these changes attained the status of advanced browsers, capable of eating leaves and soft plants and able to run fairly rapidly and for sustained periods over hard ground. With the advent of Miocene times there was a branching out of horses along several lines of development, probably as a response to an increase in the variety of environments available to them, and especially because of the spread of early grasses and other flowering plants.
An illustration on page 364 makes something of an attempt to reflect this visually, following the phylogeny of R.A. Stirton (see above) but in a more subdued and compressed manner. Being that only the genus names are mentioned, Colbert’s tree looks especially bare, although it must be conceded that it is a more accurate depiction of horse evolution than Romer’s. The illustration on page 148 of the 1961 paperback edition of G.G. Simpson’s Horses more closely follows Stirton’s phylogeny as well, and the plates likewise show the branching of tooth shapes and other characters rather than grouping forms separated by large expanses of time. The relatively rich fossil record of horses would be important to Simpson in another way as well; in his Neo-Darwinian Synthesis-era work, Tempo and Mode in Evolution (1944), Simpson was able to conclude that horses in general seemed to evolve faster than unrelated groups of animals like ammonites but more slowly than mammals like elephants. Although his hypothesis of a near-constant, albeit accelerated, rate for horse evolution has not held up today, the idea that evolution can occur more quickly or more slowly was a very important idea, an idea that took new form in Eldredge & Gould’s hypothesis of punctuated equilibria decades later.

From McFadden, Bruce. 2005. “Fossil Horses – Evidence of Evolution.” Science Vol. 307. no. 5716, pp. 1728 – 1730
So what of our current understanding of horse evolution? As I had mentioned earlier, one of the foremost authorities on the topic is Bruce MacFadden, and in 2005 he authored a straightforward summation of the current state of things in an article entitled “Fossil Horses – Evidence for Evolution.” As MacFadden notes, the overall “look” for the tree, featuring lines that did not leave modern descendants, hasn’t changed much since the time of Stirton and other earlier scientists. There has been much shuffling around and plenty of new discoveries, however, and although the diversity of late horses often gets the most attention it is now being revealed that early members of the horse lineage had a wider diversity as well. It almost seems like there’s an evolutionary bottleneck during the Oligocene, with the beginnings of more diversity in the Miocene, Mercyhippus once again leading the charge on to later forms.
MacFadden also takes a moment to correct a common misconception about horse evolution; there was no unalterable progression from small to large consonant with Cope’s Rule;
Although the 55-My old fossil horse sequence has been used as a classic example of Cope’s rule, this notion is now known to be incorrect. Rather than a linear progression toward larger body size, fossil horse macroevolution is characterized by two distinctly different phases. From 55 to 20 Ma, primitive horses had estimated body sizes between ~10 and 50 kg. In contrast, from 20 Ma until the present, fossil horses were more diverse in their body sizes. Some clades became larger (like those that gave rise to Equus), others remained relatively static in body size, and others became smaller over time.
Still, our current understanding is incomplete, and further fossil finds will continue to rustle the branches of the evolutionary bush. In fact, I would not be surprised if more early forms came to light, and I would be especially interested to see if the “Oligocene Bottleneck” is real or merely a factor of fossil collecting bias. There should be no mistake about the amazing entanglement of branches horses represent, however, and it is somewhat surprising that the public does not often hear about the true form of horse phylogeny. While I did not do an in-depth study of how horse evolution was portrayed in the popular media, from what I have seen it seems that past scientists and authors have often opted for simplicity, getting the public to accept evolution has occurred being more important than giving them an accurate depiction of how evolution works. This is a harsh lesson that we are still learning, as inaccuracies in books, museum displays, and other outlets can leave the door open for creationists to spread distrust of science. It is not enough to merely present someone who is unfamiliar with evolution with our “best” example of anagenesis; if we do, it should be in context with the larger theme of unity and diversity of forms, not a throw-away that is supposed to dazzle in and of itself. The evolution of the horse, in fact, is a perfect example of evolution and can be an extremely powerful tool in education if used properly, but for whatever reason the common theme so far has been for many popular science writers and educators to fall out of the saddle.
Evolution is truly an amazing phenomenon; who would have ever conceived of a small, four-toed animal giving rise to Black Beauty? Our overall conception of “more” being better may even make such a move from four toes to one seem counterintuitive, yet the evidence (from fossils to that of development) is clear in its implications. Horses did not spring up out of the ground from the dust, nor were they “spoken into being” by an omnipotent power that decided that Adam should have a faithful steed. Every bone in their body cries out as to their past, and we are all the more enriched to understand that just like the horse, Homo sapiens is a still-changing product of a long and rich evolutionary history, too.
I’ve been working on a brand new historical/evolutionary review post all day, and I’m sad to say it’s not yet completed. I was expecting a finished product by now, so as a substitute, here’s the first few paragraphs of the post that should give you some clues as to what I’ll be covering. I haven’t gone back to fully edit yet so please excuse any errors. I hope to have the whole thing finished tomorrow (it’s currently a little more than halfway done);

One of Charles R. Knight’s interpretations of Eohippus
When the name of O.C. Marsh is invoked, it is often to tell of his participation in the great “Bone Wars” of the late 19th century, sparring with fellow osteophile E.D. Cope in the pages of the New York Herald. Twisted tales of deceit and sabotage were promulgated in the sensationalist paper, and while both men helped to bring about an American revolution in vertebrate paleontology, the scars of their bitter squabbling have yet to fully heal. Such scientific in-fighting might seem worthy only of a historical footnote or an introduction to the stereotyped image of “smash-and-grab” paleontology of the time which is almost romantically referred to, but the truth of the matter goes far deeper than the public beard-pulling that is so often remembered.
The tiff between Cope and Marsh is strange in that is seems to exist in the popular literature out of time, removed from the context in which it had originally existed. Charles Darwin had published his earth-shaking work On the Origin of Species by Natural Selection a scant 31 years before the ink almost ran red with rage on the pages of the Herald, the question of evolution being of far more importance in the public consciousness than dinosaurs. The full establishment of the dinosaur as a cultural (and dare I say, mythical) creature in the mind of the American public only seemed to take place after the Bone Wars, the appointment of Henry Fairfield Osborn to the American Museum of Natural History (specifically hired to establish a vertebrate paleontology program) and the popular reports of the dinosaur that carried the namesake of Andrew Carnegie, Diplodocus carnegei, being the more immediate beginnings of the public’s love affair with the extinct creatures. Before Brontosaurus and Tyrannosaurus became household names, the public eye was focused upon horses and birds.
The latter half of the 19th century was a stirring time for biological science, especially involving the new areas of vertebrate paleontology and evolution, the august authorities in England keeping on eye on the up-and-comers starting their own careers in the states. Early on, paleontologist E.D. Cope impressed T.H. Huxley with his 1866 discovery of Laelaps aquilunguis, but in a paleontological clean-sweep Marsh would eventually have his name attached to Cope’s dinosaur and the admiration of not only T.H. Huxley, but Charles Darwin himself. As for the renaming of Laelaps, Marsh found that the name was already taken by a genus of mite, renaming the New Jersey greensand dinosaur Dryptosaurus in 1877 (although Cope, throughout the rest of his career, called the dinosaur Laelaps). It would take more than some taxonomic shuffling to impress the eminent British anatomists and paleontologists, however, and Marsh’s ticket into Huxley’s good graces came in the form of toothed Cretaceous birds like Hesperornis (Marsh, 1872).
Here’s the last set of the photos from this past weekend, and as soon as I get the entire set uploaded to Flickr I’ll let you all know.

One of the gorillas at the Primate House. I only saw two while I was at the zoo, so I assume that most of the rest of them were inside or in an area out of view.

A Grevy’s Zebra (Equus grevyi).

A Prairie Dog (Cynomys sp.) having a snack.

The only visible “enrichment” the African Elephants had was a naked metal chain, and the elephant that I was told was named “Petunia” couldn’t stop fiddling with it. I have to wonder if some of this was a way to comfort herself or divert unease, as just before this picture she was driven out of the shade by the two other elephants she shared her habitat with, and overall she seemed to be restless/ill at ease the entire day.

This is “Puzzles,” the Reticulated Giraffe (Giraffa camelopardalis reticulata). I don’t know why the zoo staff has not operated on this animal, and the plaque outside the enclosure is a bit shore on details. Perhaps the condition is inoperable, and it doesn’t seem to visibly inhibit the giraffe, but I do feel sympathy for Puzzles.

The most deadly of all creatures, the Pygmy Marmost [Callithrix (Cebuella) pygmaea]. Don’t let their cute appearance fool you; they can deskeletonize a cow in seconds… or is that piranhas…

A Sable Antelope (Hippotragus niger) rests in the shade. It’s hard to tell from this angle, but this one has asymmetrical horns.

A Squirrel Monkey (Saimiri sp.) enjoying some fruit.

A Tree Shrew (Tupaia sp.), sitting still long enough for me to squeeze off a shot (albeit a blurry one).

One of the Galapagos Tortoise (Geochelone nigra) taking a dip.

A pair of Aardvark (Orycteropus afer) having a snooze in an artificial cave.

“Solstice,” a female White-Handed or Lar Gibbon (Hylobates lar). She shares her habitat with her partner Mercury (who is black rather than blonde) and an Orangutan (Pongo sp.) pair.

Solstice makes her intentions clear; she wants to be groomed by Mercury.

Mercury seemed more interested in grooming his male orang friend than his partner Solstice.

The male Orang was very shy, and carried a cardboard box on his back like a shell/shade everywhere he went.

And that’s it. Hopefully I’ll soon upload all my photos (there are thousands of them) onto Flickr soon, but I hope that you’ve enjoyed the ones I’ve put up here.
As promised, here is the second set of the better photos from my trip to the Philadelphia Zoo. I probably should (at the reccomendation of several commentors) register with Flickr and upload the lot of them, but that will have to wait until tomorrow (I’ll also go back and do likewise for the pictures on this computer as time permits). Let’s pick up where we left off, with one of my most favorite of big cats, the Amur Leopard (Panthera pardus orientalis);




There is nothing quite so beautiful as the emerald, fiery stare of an Amur Leopard. The eyes of almost any big cat can be described as intense or as being as intricate as a precious stone, but there is something about the gaze of leopards that strikes me in an entirely different way than that of their cousins…

…yet even the most majestic and feline predators needs to make time for a brief tongue-bath every now and again.




It’s amazing the amount of bravado an inch or so of glass can produce. The object of the leopard’s stare was a child that could not have been more than two years old, being held up to the glass by his parents to get a closer look at the “big kitty.”

At times the leopard seemed just as interested in what I was doing as I was in his activities.

It is sad enough that this leopard is among the last of his kind anywhere in the world, being the most endangered of all the big cats. Why he is left on display in isolation, with not even as much as a plaque explaining what species he is and the problem those still in the wild face, not to mention the (as far as I can ascertain) the lack of a breeding/conservation program, confuses and frustrates me.


The Giant River Otter (Pteronura brasiliensis) were also released just as my wife and I reached their enclosure. They certainly seemed excited to be out in their habitat, full of fish for them to snack on.

At one point something apparently spooked the group, and they engaged in a “mobbing” behavior similar to that seen in the BBC’s Planet Earth series when a group of otters of another species faced a Mugger Crocodile. What the disturbance was, I couldn’t tell, but it seemed to come from the other side of their enclosure.
Unfortunately WordPress was a down for a little while last night so I didn’t get to upload the rest of the pictures, but I will do so during a break between my classes in a few hours. Snuggling Aardvarks, primates (from prosimians through apes), and mammalian herbivores of various description.
As promised, here are some of the better shots from yesterday’s visit to the Philadelphia Zoo. I’m sorry to say that I’m going to soon write up something about the Zoo’s shady dealings involving it’s African Elephants (visit Help Philly Zoo Elephants for a spoiler), but for now I’m going to focus on some of the better photos out of the 500+ I shot yesterday. And away we go…

I absolutely love this fountain.

While not particularly exotic, Scottish Highland Cattle are still pretty neat.

A pair of rare Blue-Eyed Lemur, Eulemur macaco flavifrons. The black one is the male, the blonde the female, and they were very excited at the prospect of a snack (the mangabey next door was getting ded fed at the time)

One of my most favorite of all mammals, the Giant Elephant Shrew (Rhynchocyon petersi).

This, by far, was the thinnest Mara (Dolichotis sp.) I think I have ever seen.

The Galapagos Tortoise (Geochelone nigra) were just beginning to stir when we arrived. They weren’t nearly as randy as they had been during our last visit (I thought I had heard it all until I hear the deep tones of tortoise-lovin’)

An African Elephant (Loxodonta africana) that we were told was named “Petunia” was also up and about. The Philly elephants will soon be moved out of their rather meager accomodations, although it might not necessarily be for the better.

This little male Amur Tiger (Panthera tigris altaica) really loved his tire. He wouldn’t let any of his brothers near it without showing his annoyance.




The strangely white female lions were relaxing in the early-morning shade. I know that their condition is a regional variation, although I forget the details at the moment.



Some of my most favorite Carnivores, White-Nosed Coati (Nasua narica) were scrounging for insects and other morsels when we passed by their enclosure.


And, just for Jeremy, a Red Panda (Ailurus fulgens).

We also came across the most evil-looking Caiman I had ever seen (there was no ID plaque, so I’m not sure what species it was).

And the Clouded Leopard (Neofelis nebulosa), as ever, was asleep in it’s hammock. I have never seen this cat move a muscle in my four visits to the Philly Zoo thus far.

Just around the corner, however, was a much more active and curious cat; a male Amur Leopard (Panthera pardus orientalis). He is one of the most beautiful big cats I think I have ever seen, and it’s a shame that he’s essentially “locked up” in his enclosure, and as far as I know the zoo does not keep a female Amur Leopard to run a breeding program for this most critically endangered cat.

I still have at least 25 pictures to share, but you’ll just have to wait a little bit longer for them. Check back later tonight for more of our friend the Amur Leopard, some Giant River Otter, White-Handed Gibbons, and plenty more.
Many of the world’s great natural history museums devote at least one hall to creatures that no longer exist today. In the old tradition, in order to keep any young upstarts from getting any ideas about evolution, skeletons or parts of skeletons were grouped by the functions they performed, a visitor being likely to find the wing of a bat and the wing of the bird in the same display case even though the two animals extremely distantly related. Newer layouts, conversely, have largely ignored the end-function of one line or another to group animals together by homology and their shared characters, the most well-known example being the remodeled 4th Floor of the American Museum of Natural History in New York City which has attempted to arrange its fossil collections as a walk-through cladistic diagram.
Still, the generally discarded of grouping animals by their adaptations to general habitats or niches is not without it’s charms. Over and over again, evolution has produced forms that seem to converge on certain body plans, varying habitats making some traits advantageous and others a liability, helping to adapt different organisms to their local ecologies. Flight has independently evolved several times (and the ability to glide an even greater number of times), as well as adaptations to marine environments, saber-like canine teeth, immense sails along the spine, and slicing premolar teeth, although each time such familiar features seem to arise it shows that there is more than one way to solve an evolutionary problem from any given point in an organism’s natural history. Not everything can be chalked up to convergence of form in order to carry out particular functions, however. Parallel evolution, although sometimes difficult to determine, also allows relatively closely related forms to take the same evolutionary paths, showing many of the same anatomical characters even though they diverged from a common ancestor at some point in the past and occupy at least two different lines of descent. In fact, it is often these weird and wonderful creatures that are forgotten or overlooked, more people recognizing the term “saber-toothed cat” (or, loathe as I am to say it, “saber-toothed tiger”) or the genus Smilodon than the term “Nimravid” or the genus Dinictis. The following entry, therefore, will be an attempt to navigate through the somewhat “entangled bank” of evolutionary relationships among animals that appear to be shaped in similar ways by the environment but constrained by their species’ history, showing us that there is more than one way to make a saber-toothed cat.
Back into the pool: Of Ichthyosaurs, Sharks, and Cetaceans
Perhaps one of the most well-known (or at least widely cited) examples of evolutionary convergence has been that of the similar body shapes of sharks, ichthyosaurs, and cetaceans. It’s difficult to see these three distinct groups of creatures side by side and not recognize the similarities, but why are they similar in the first place? If they belong to groups that are distantly-related branches of the evolutionary “bush,” why should they have developed similar body forms?
One of the most well-known examples of evolutionary convergence; (From Top to Bottom) An ichthyosaur Ophthalmosaurus icenicus, a Porpoise, and a Spiny Dogfish (Squalus acanthias)
From the 1925 creationist book The Predicament of Evolution by George McReady Price.
Creationists have been quick to seize upon the idea of convergence as if it were one of evolution’s weak points. In 1926, George McCready Price wrote the following in one of the more well-known early American anti-evolution texts, The Predicament of Evolution;
For instance, we have the shark, the ichthyosaur (an extinct kind of fish-shaped reptile), and the dolphin (a true warmblooded mammal, and not a fish at all), all of which greatly resemble each other in external shape and general appearance. Each has the same long, sharp snout, the same powerful tail, the same general fishlike shape. And yet the first of these is a true fish, the second was just as true a reptile, while the third is a mam-mal, bringing forth its young alive and feeding them by milk, just as does a cow or a horse, though it lives in the sea.
Here the evolutionists have to say that this peculiar shape and general form has been evolved separately and independently in each of these three instances. Indeed, Henry Fairfield Osborn, President of the American Museum of Natural History, New York City, declares that a very similar shape and form has been independently evolved “at least twenty-four times.”—”Encyc. Brit.,” Vol. XX, p. 578…
From this large group of facts we become convinced that these many similar or identical structures, which must have been evolved quite independently (if evolved at all), make too great a draft on our credulity. At least, these hundreds of examples of “parallel evolution” greatly weaken our confidence in homology, or similarity of parts and organs, as a proof of blood relationship.
Such arguments have become traditional amongst creationist apologists, suggesting that if convergent evolution does occur then we must throw homology out the window as similar structures will only mislead us as to the true affinities of the creatures being studied. As we will later see with Cuvier’s Ptero-dactyl, this can be a danger for scientists who are unwary and wish to shoehorn creatures into existing taxonomic categories, but not for those who actually look beyond superficial appearances.
The reason why the shark, the ichthyosaur, and the porpoise should all look vaguely the same is because they live(d) in the same environment; the ocean. An organism that is suspended in a fluid that is much denser than air can be adapted in various ways to such an “alien” environment, but physics does dictate what shapes can be taken based upon life history. It is possible to be a floating filter feeder, exhibiting a round shape, but such a strategy is essentially out of the question for animals that need to move quickly and to hunt for food. What is required is not only a powerful propulsive organ to keep the organism moving forward, but also extra appendages to allow for the control of movement and a streamlined shape to reduce drag (and hence reduce energy costs for moving through the water).

One of Charles R. Knight’s renditions of an ichthyosaur.
In fact, sharks as a whole provide a good model for various forms of ichthyosaurs. While ichthyosaurs are generally presented as already being streamlined and possessing a large caudal fin with two equally long lobes, we would be loathe to forget that they too are products of evolution and many fossils show us that they were not always an Euryapsid (thank you, johannes) answer to modern-day Lamnid sharks. Early ichtyhosaurs actually had more of a “bump” towards the back of their tail rather than a full-blown caudal fin, their overall body shape and lack of a large propulsive surface keeping them from moving too quickly through the water. A similar tail type/form can be seen in many modern day sharks like the Nurse Shark, which generally live along the bottom feeding on crustaceans and inhabitants that can be sucked out of coral crevices. Being that ichthyosaurs lack gills, it is unlikely that their early representatives were bottom-dwellers, instead preferring shallow areas, which can be especially productive in terms of food.
Modification of the “tail kink” (which was at first thought to be a taphonomic feature, early reconstructions showing “amphibious” ichthyosaurs with straight tails) seen in early forms allowed for the eventual evolution of a crescent-moon shaped tail, as well as adaptations in the skull and of the limbs into fins (the addition of digits and the addition of bones in the digits being quite common in the latest forms). This more-familiar shape would allow ichthyosaurs maximum propulsion with their caudal fin (the spine going downwards instead of upwards, as in sharks) while they would be able to exert control over their motions with their pectoral fins and would be kept from rolling in the water by their dorsal fins. The evolution of large eyes and other features aside, the overall shape and basic skeletal structure of ichthyosaurs seems to be an optimal design for medium-to-large, fast-moving, oceanic predators (although mosasaurs, pliosaurs, and plesiosaurs took different evolutionary routes).
What allowed ichthyosaurs to develop an effective side-to-side motion of the tail would not work for cetaceans, however. Ichthyosaurs developed their mode of propulsion by side-to-side motions of the spine, perhaps swimming in a mode similar to eels or cat sharks at first, a common form of locomotion in modern reptiles. This sort of motion is usually accomplished on land via a sprawling gait, the limbs being held out to the sides and the animal exhibiting a bit of a side-to-side motion as it moves along.
Whether the ancestors of icthyosaurs were sprawlers (to a greater or lesser extent, predisposing them to side-to-side motions of the tail and body) or not, cetaceans evolved much more recently in evolutionary history, and developed from ancestors that carried their legs directly underneath their body. The plasticity of early archaeocetes and their artiodactyl ancestors was greatly diminished, their hip and spine structure adapted to up-and-down undulations rather than the side-to-side motion seen in the video of the salamander. This sort of constraint has not stopped mammals from becoming adapted to the water, however, and clues to the evolution of cetacean movement can be seen in living animals like Giant River Otters;
In the water, undulations of the spine accompanied with some propulsion from the limbs proves to be very effective, and it’s not hard to imagine an archaeocete like Ambulocetus, as my friend Neil so aptly described, as a “sexy otter.” Once undulation of the spine became established as a method of moving through the water, the eventual addition of a tail fluke would do for cetaceans what the crescent-shaped tail of tuna, sharks, and icthyosaurs acheived in terms of speed and power, the body being adapted towards a streamlined appearance with (again) the pectoral fins providing lift/control and the dorsal fin preventing rolling. Larger forms of whales, namely the Mysticetes or Baleen Whales, grew to immense size and gave up some of the features that seem to be convergent with sharks and the smaller ichthyosaurs (some, in fact, did acheive whale-size), but they are derived from more predatory designs and their niche as massive, far-ranging suspension feeders free them from some constrains while imposing some new ones.
A painting of leaping ichthyosaurs by Heinrich Harder (circa 1916)
Human engineering has recognized similar constraints for motion in the water and even in the air; planes and submarines most closely resemble sharks and dolphins in overall shape, the placement and size of the wings on a 747 having much the same function as the large pectoral fins of far-ranging pelagic fish like the Blue Shark. Life in the water adapted all three groups of animals towards the same shape because there does not seem to be any other way to be a fast-moving, medium-to-large sized marine predator; speed and some degree of maneuverability are paramount. Some other lines have diverged from this shape, as noted before, but the sharks, dolphins, and (I don’t think it’s too much of a stretch to say) ichthyosaurs all occupied essentially the same niche and therefore were adapted in a particular fashion.
Do not think, however, that the convergence of three lines towards one body plan gives credence to a kind of “orthogenesis” or progressive force driving evolution. There was no sort of supernatural or external force manipulating the genetic material of these groups with the shape of a dolphin or shark in mind. Rather, the environment and local ecology determined what form would be favored through time, and even though the three groups may look the same and have significant convergences, they also have many traits in common with their ancestors, allowing us to trace their evolutionary history (which is why no one is arguing that dolphins, sharks, and ichthyosaurs are closely related or form a small monophyletic grouping).
A marsupial you wouldn’t want to meet
Living members of the Carnivora (bears, cats, dogs, civets, weasels, etc.) have always caught my attention, but there was an entire group of carnivorous mammals, now extinct, that have left no living representatives. The last known member of this group was named Thylacoleo carnifex by Richard Owen, and it has some of the strangest dentition ever seen in a marsupial. Marsupial mammals are well-known in Australia, creatures like kangaroos, koalas, and wombats coming most immediately to mind out of living extant taxa. There was a much more diverse population of marsupials during the Pleistocene, however, and the “marsupial lion” was likely a formidable predator.

A skull of Thylacoleo on display at the AMNH.
In order to understand why Thylacoleo is relevant to our discussion of convergence we need to first understand what makes living placental Carnivores so special. Many carnivores, especially cats, have a rather specialized dentition, certain molars and premolars making up what is known as the “carnissal shear.” These teeth are pointed and act like scissors, easily cutting up flesh or crushing bone. The molars behind the shear are often reduced (some groups have retained their molars in order to incorporate a more generalized diet, like dogs and bears), the dental specialization perhaps being one of the keys to the success of this group. Earlier predators of now-extinct lines like Mesonychids lacked such specialized cutting teeth, and the teeth behind the canines of the large Andrewsarchus show that their oral tool-kit was a bit more blunted.

The skull of Andrewsarchus, on display at the AMNH
Thylacoleo, a carnivorous marsupial not descended from the Miacids that gave rise to living carnivores, also developed something of a “carnissal shear” but in a different way. Rather than a battery of teeth that became sharpened, one of the upper and lower premolars of Thylacoleo became elongated and blade-like, and the cleaver-like teeth helped to sharpen each other as they moved past each other when opening or closing the jaw. Thylacoleo also had a terrible bite, the attachments for the muscles that opened and shut the jaw were massive, somewhat constricting the amount of space the brain could take up, but giving Thylacoleo what was perhaps the most powerful bite forces amongst mammalian predators, especially given it’s relatively small body size (it was only about four feet long and 220 pounds).
Thylacoleo is an odd marsupial in another respect; the claw on its thumb was retractable like that of a big cat. This sort of adaptation is especially useful in keeping claws sharp, and perhaps keeping the claws sharp would allow Thylacoleo to get a good hold on its prey before going to work on it with its teeth. At this point I should probably mention that some scholars in the past have thought that Thylacoleo was an herbivore, not unlike the extant marsupial Phalangers. I will leave the response to such an argument to Richard Owen;
These eminent authors received the support, in reference to objections to my conclusions, of the (then) Curator of the Australian Museum, Sydney, Mr. GERARD KREFFT, who, in his contribution to the ‘Annals and Magazine of Natural History,’ series 3, vol. 18, 1866, p. 148, records his opinion that “the famous marsupial Lion was not much more carnivorous than the Phalangers of the present time.”
The species of carnivorous Phalanger is not named. No evidence of such by fossil specimens has reached me, nor have I found such exceptional habit of an existing species of Phalangista elsewhere noted.
As my friend Zach has noted, however, calling Thylacoleo a “marsupial lion” is a bit misleading. Even though some lion-like aspects of the skull (the results of convergence on a hypercarnivorous lifestyle, and Thylacoleo means “pouched lion”) led the anatomist Richard Owen to name the creature on the basis of such resemblances, the ways in which Thylacoleo shows its marsupial affinities are much more important. Referring to this animal as the “marsupial lion” without qualifications (as well as calling the extinct Tasmanian Tiger the “marsupial wolf”) usually confuses more than illuminates, and creationists often take the names and superficial resemblances to mean that evolution didn’t occur. Instead, they propose that God made the beginning of a “kind” of carnivorous mammal which was preserved on Noah’s Ark and gave rise to all later forms, important reproductive habits deemed to be of little consequence.
Even so, Thylacoleo carnifex and its relatives represent a branch of marsupials that became fairly specialized predators, and given the plasticity of tooth structure, it’s not hard to see how sharp premolars could be adapted into a blade to cut flesh. While it may be easy to draw connections between this animal and living carnivores, however, perhaps we should be more measured in our descriptions; both groups met the same challenges in similar ways, but the differences are far more striking and important in this example of convergence on a particular niche.
On what day were the Ptero-Bats created?
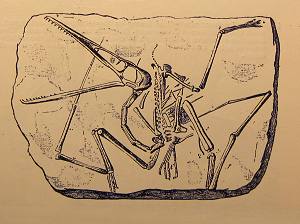
An engraving of the creature now known as Pterodactylus antiquus, the very one described by Collini.
Before there were natural history museums, there were motley assortments of organic odds and ends known as curiosity cabinets, and in the cabinet of Karl Theodor there would eventually come to be a petrified treasure. Although it was probably collected around 1767, the first known pterosaur fossil was not described until 1784, when the appointed caretaker of the collection, Cosimo Alessandro Collini, attempted to determine the nature of the strange creature that came to him from the limestone of Bavaria (the same deposits that later yeilded Archaeopteryx). Although certain that he was the remains of an animal from an earlier time, Collini was agnostic about what kind of animal he had come to possess. Years later, the famed anatomist Georges Cuvier investigated Collini’s paper and illustrations, noting that the creature was certainly a reptile. Still, the fossil would remain without a proper name until Cuvier would write a more detailed analysis in 1809, dubbing the fossil “Ptero-dactyle.”
Not everyone agreed with the analysis of Cuvier, however, especially since Cuvier did not get to see the fossil himself and had to work from the drawings in Collini’s paper. Samuel Thomas von Soemmerring, of the Bavarian Academy of Science, thought that the pterosaur was some unknown type of bat, a view that would remain entrenched in the minds of some scientists for many years. Indeed, one restoration by Edward Newmann in 1843 (and “re-drawn” for Gosse’s work Omphalos, as shown below), depicted the two known types of pterodactyl known at that time as fuzzy bats, complete with cute little ears. It is clear from the drawing that pterosaurs do not make good bats, although this didn’t stop many German paleontologists from taking such a stance through the first half of the 19th century.

Newmann’s “marsupial bats”, conspicuously missing their ears, from Gosse’s Omphalos. It’s likely that Gosse recognized the reptilian nature of these Pterodactyl by the time he wrote his book, so Newmann’s work was copied minus the more mammalian aspects.
But why was there such confusion? It is likely because there is something familiar about pterosaurs that had been seen in living bats; the extension of digits to hold a membraneous wing. While the first fossil, despite wonderful preservation, did not preserve a membrane impression, it is hard to look at it and not recognize the superficially similar structure of a bat’s wing, which also carries a membrane to enable flight. In fact, birds seem a bit unusual in developing feathers for flight; many varieties of gliding and flying creatures have taken to the air (regardless of whether they engage in powered flight or glide) by the use of membranes. Indeed, gliding may often precede powered flight, and once an animal has developed a membrane that can be stretched between its limbs to glide, the extension of the digits at the point(s) of attachment can help to expand the wing size. Such changes likely occur as a result of changes in development, natural selection favoring the invasion of a new niche based upon variations that exist in a population, although in the case of pterosaurs we can no longer test to see if this is correct.
As we just saw with Thylacoleo, however, the convergences of pterosaurs and bats are rather slight, overall. While both acheived flight on membraneous wings attached to extended digits (many more in the case of bats) and have relatively compressed bodies, pterosaurs had a much greater diversity in shape and size than modern bats. Likewise, they did not elongate the rest of their fingers, suggesting that there was some situation (be it climbing or hanging on to a perch) that the pterosaurs still needed their other fingers for (although bats can climb pretty well with their thumbs, and some have even evolved suction disks). Still, it can be said that both took to the air by similar means and had to deal with similar constraints, but their evolutionary paths are far more divergent than that of the aforementioned sharks, ichthyosaurs, and cetaceans.
It doesn’t look like much of a planet-eater to me

A female Gharial at the National Zoo in Washington, D.C.
Perhaps one of the most unnecessarily confusing groups of extinct animals are the phytosaurs. Filling the niche now occupied by reptiles like the Saltwater Crocodile, the water-dwelling archosaurs have left no living descendants despite their past diversity. At first glance, the phytosaur Rutidon looks just like a modern-day Gharial, and even though it shares a common ancestor with the reptiles that now exist in tropical watery habitats all over the world, it is not otherwise related. The most prominent phytosaur feature is that their noses are over or just anterior to their eyes on their head, not at the end of their snouts. This would allow them to breathe while completely submerged, although their eyes might not have been above water when hiding in such a manner. Even beyond this feature, their jaws seem to be fairly simple, merely having a hinge at the back to open-and-close. Compare this arrangement, here represented by the giant Machaeroprosopus gregorii, with the more complex reconstruction of the true crocodilian Deinosuchus (although, admittedly, this reconstruction was heavily based upon the living Cuban Crocodile and may not be fully accurate. It still serves to show the differences between the groups, however).
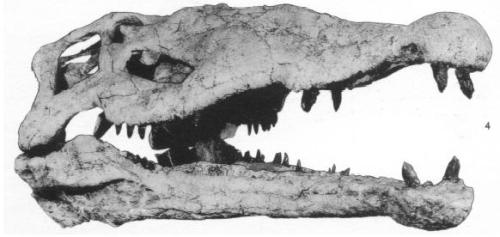
Machaeroprosopus, currently on display at the AMNH
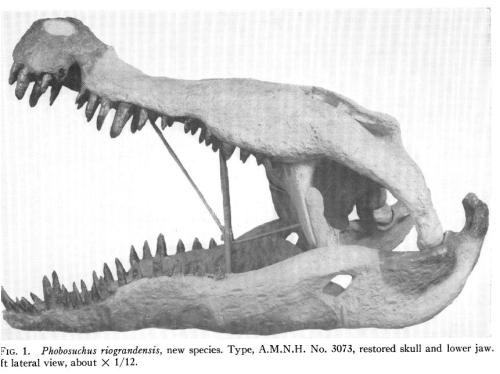
Deinosuchus reconstruction, formerly on display at the AMNH
The most notable difference are the complex bones at the back of the throat of Deinosuchus which are arranged to slide past each other as the jaw opened and closed. No such feature is seen in the giant phytosaur. Still, even after the phytosaurs died out, crocodilians did not return to the water until about the Cretaceous period, many forms being absolutely terrifying land predators that have also long been extinct. One of the early forms was Protosuchus, a small true crocodilian that represented a line that changed little during its tenure on the earth.
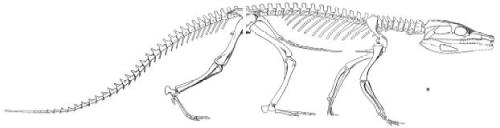
Reconstruction of Protosuchus
Outside of walking relatively high off the ground, Protosuchus had a foreshortened snout which was lower than its eyes, quite different from the arrangement in living crocodilians. As seen in the Dwarf Caiman photograph, below, living crocodilians have their eye sockets on the top of their head, their eyes sticking out on the surface as well as the tip of their nose when they lie in wait for prey (or just rest, for those who would like a less sensationalist tone). Protosuchus, by contrast, has eyes to the sides of the head, even facing somewhat forward, showing that it was much more well-adapted to the land than any swamp or shallow pool. Crocodilans did eventually enter the water, however, and their fossils are among the most common of any vertebrates. Some, like New Jersey’s very own Thoracosaurus, even became marine species, and a few varieties evolved crescent-shaped caudal fins on the ends of their tails to help them swim. The common belief, however, is that crocodiles have always been crocodiles, “changing little since the time of the dinosaurs,” and such generalized half-truths do little justice to crocodilians or their distant phytosaur cousins.

Saber-toothed Nimravid doesn’t sound quite the same…
Many museums have cases devoted to the great saber-toothed cats of epochs long gone, but it would take someone with more than just a cursory understanding of paleontology to sort out what is really being displayed. Saber-teeth, or elongated canines, have evolved many times over in the course of mammalian history, showing up in herbivores like the living Musk Deer as well as extinct groups like the gorgonopsids. Animals as different as a Musk Deer and Inostrancevia are fairly easy to tell apart, even for the non-specialist, but what about nimravids and the “true” saber-toothed cats?
A diagram of the three ideas of Nimravid/Felid evolution.
James Whitcomb Riley is purported to have once written “When I see a bird that walks like a duck and swims like a duck and quacks like a duck, I call that bird a duck.” Unfortunately, this argument is quite popular (even being utilized by the likes of prominent Intelligent Design advocate Michael Behe) despite being very superficial and even vapid. Needless to say, it doesn’t apply to our discussion of Nimravids and true felid saber-toothed cats, but in decades past the two groups were lumped together.
<img src=” ” alt=”Skulls” />
” alt=”Skulls” />
So, what makes a nimravid a nimravid? They look awfully like cats, so why aren’t they included in the Family Felidae? What makes such distinctions so difficult is that those investigating the skull of Smilodon and Eusmilus would have to be relatively well-versed in scientific jargon and anatomy in order to point out the most important differences. While some nimravids (like Eusmilus) had large canines, their teeth alone are not diagnostic, and the original factors used by E.D. Cope that differentiated these animals from “true” cats were the “alisphenoid canal, postglenoid foramen, carotid, posterior lacerate, and condyloid foramina, postparietal foramina” in the skull (Hunt, 1987). The various canals and foramina listed dictate the paths of various nerves and blood vessels in the skull, and the arrangement in nimravid skulls seem to be more primitive compared with true felids. Likewise, nimravids lack a two-chambered auditory bulla, which is a rounded bit of bone associated with the ear which true cats posess.
There are a few more obvious giveaways when dealing with some nimravids, however. Nimravids equipped with long canines often have more cone-shaped canines than those of saber-toothed cats (which are flatter in cross-section), and many have bony “sheaths” extending from the lower jaw into which the massive teeth fit. Perhaps the most famous example of this kind of arrangement is the genus Barbourofelis, an animal that has actually been assigned to its own family as it is likely more closely related to true cats than nimravids (Barbourofelis was previously classified as a nimravid). Because of this (and the fact that another cat-like offshoot, the marsupial Thylacosmilus) the tooth-sheath shouldn’t be considered diagnostic of nimravids only, but it does give you a substantial clue that you’re probably not dealing with an actual saber-toothed felid.
Despite these differences, it has often been difficult to differentiate the groups (and debate still continues). The diagram above, based upon one in Robert Hunt’s 1987 paper “Evolution of the aeluroid Carnivora. Significance of auditory structure in the nimravid cat Dinictis,” offers three simplified versions of the hypotheses about the relationships of nimravids and felids. Initially it was thought that there was a progressive evolution from ancestor to descendant in a straight line, the nimravids being the direct ancestors to the saber-toothed cats. This view does not represent how evolution truly works, however, and was found to be incorrect. In its place came a view that nimravids and saber-toothed cats diverged from a common ancestor at about the same time, going off in separate directions. This is better, and is more consonant with the data, but again it suggests that the line representing the common ancestor went extinct, either in becoming nimravids or saber-toothed cats. What seems to be the case based upon current data is that the nimravids split off from a common ancestor somewhat before the saber-toothed cats, the line containing their common ancestor continuing its own evolution as both groups evolved. Such a branching pattern is not unusual, and should even be expected, especially since there are living primates like tarsiers and lemurs that represent the overall kind of animal our ancestors once were, but still quite different and undergoing their own evolution alongside our own lineage.

The skull of Thylacosmilus, the marsupial answer to the saber-toothed cat, on display at the American Museum of Natural History in New York City. Note how far back in the skull the roots of its massive canines extend.

The skull of Megantereon on display at the AMNH. It was one of the “true” saber-toothed cats.
To complicate things even further, the skull or skeleton of the marsupial Thylacosmilus is also often thrown into the mix. Although totally unrelated to nimravids or felid saber-toothed cats, the South-American Thylacosmilus converges closely on the appearance of the placental predators, although there are some important differences. As can be seen from the above photographs, the eye of Thylacosmilus is entirely enclosed by bone on the side of the head, while in many felids and nimravids the eye socket is not entirely ringed-in by bone as if someone had bored a hole in the skull (compare the skull of Thylacosmilus with that of Thylacoleo, above). Further, the teeth of Thylacosmilus have very deep roots, going back in the skull almost over the eye. Originally it was thought that the teeth faced outward, but this was based upon a distorted skull and later finds showed the true position of the long canines.
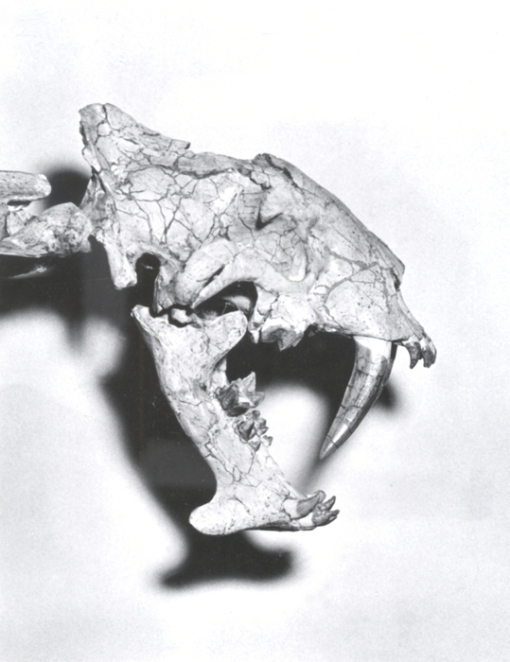
Hoplophoneus
Now that we have elimated Thylacosmilus from the running as another case of marsupial convergence, we must ask why the nimravids and felid saber-toothed cats are so close to each other in appearance. While many of the instances I’ve discussed previously have been instances of convergence, be it throughout the entire body or merely certain aspects of it, the nimravid-felid connection is a wonerful example of parallel evolution. W.E. le Gros Clark provides an excellent summation of understanding the difference in his 1959 book The Antecedents of Man;
From what has already been said, it is clear that, in assessing degrees of phylogenetic affinity, it is always necessary to take into account the factors of parallelism and convergence in the evolutionary development of related or unrelated groups. These processes can lead to structural similarities, which, taken by themselves, may be misleading. The term convergence is applied to the occasion in general proportions or in the development of analogous adaptations in response to similar functional needs. But such similarities are superficial and easily distinguishable by a detailed comparative study of the animal as a whole. For example, the resemblance in general appearance, even in a number of morphological features, of the Tasmanian wolf to a dog does not obscure the fact that in fundamental details of their anatomical construction they belong to quite different mammalian groups. On the other hand, the potentialities of parallelism seem often to have been much overestimated by some anatomists, for this phenomenon has sometimes been invoked in support of extreme claims for independant evolution of groups which are almost certainly quite closely related. We can agree with G.G. Simpson that the whole basis of parallelism depends on an initial similarity of structure and the inheritance of a common potentiality for reproducing homologous mutations, and that, this being so, the initial similarity and the homology of mutations themselves imply an evolutionary relationship. Expressed in another way, it may be said that convergence increases resemblances (which are, however, no more than superficial), while parallelism does not so much increase resemblances as maintain and perpetuate (by development ‘in parallel’ so to speak) similarities which have already existed ab initio in the genetic make-up of related types. Thus, ‘closeness of parallelism tends to be proportional to closeness of affinity.’
There are a few problems with this reasoning, namely that it seems to give credence to an almost pre-determined genetic course for the lines to evolve in parallel, although le Gros Clark makes it clear in the work that he does not support in any way the notion of orthogenesis. Still, the passage makes the important distinction that in order to undergo parallel evolution groups need to be somewhat closely related and already bear similar structures, evolution preserving many of the similar traits instead of working to the same end from two disparate points. In the case of the nimravids and the felids it seems that they evolved from a common ancestor which was probably taken to carnivory. Nimravids branched off earlier, being more “primitive,” while the felids came off the same line (or a very similar one) after it had accumulated a few more evolutionary changes. Indeed, even if form seems to be static or change little, it’s hard for me to believe that designs are not slightly adapted this way or that as if the creature was an already perfect creation not influenced by changing ecological circumstances. Still, it seems that the nimravids and felids were adapted in similar ways, their ancestral lines probably possessing at least semi-retractable claws, long and sharp canines (although not long to the extreme like its descendants), a shortened face, and a developed carnissal shear. It is really not that difficult to change a civet-like creature (or in the case of our hypothetical common ancestor, a creature a bit closer to a cat) into a saber-toothed Smilodon, the changes being modifications of existing structures more than the creation of something entirely new out of nowhere. In fact, the vertebrate tetrapod skeleton has proven to be quite versatile, and most of the major bones in any vertebrate skeleton can be found to correspond with those in another vertebrate, allowing us to compare rhinos with ceratopsians, dromeosaurs with birds, cats with dogs, ichthyosaurs with cetaceans, and humans with primates.
Of constraints and convergence
I hope that is has become clear why convergence is such a strong theme in the evolution of vertebrates. At this point in the history of evolution, vertebrates have had a chance to fill nearly every niche imaginable in a large variety of habitats over millions of years, and so common themes are bound to arise. When groups return to the ocean, the environmental constraints shape them in ways peculiar to their new way of life that would not be advantageous in other situtations (i.e. being such a large aquatic animal that you’d be crushed by your own weight if you came onto land). When mammals become adapted to be predators, their dentition and morphology must be altered if they are to be successful hunters, carnivores past and present showing some suprising similarities despite being only distantly related. Even when taking to the air, laws of physics still apply, and natural selection often works through physical and chemical constraints to produce new forms.
It is of little doubt that the tetrapod design is a versatile one, retaining its overall character through the various changes that it has endured. Indeed, even when a lineage dies out and may seem gone forever, there is no law that says a similar situtation in the future will not produce forms that may be strikingly familiar, even if such organisms are not directly related to the last group that filled their new niche. Evolution has produced “endless forms most beautiful and most wonderful” and will continue to do so long after I am gone, but random mutation/natural selection do not work in isolation from the rest of the natural world. Evolution has produced so many amazing creatures precisely because ecology, physics, and chemistry have offered up both opportunities and challenges, and I only regret that I will not be able to witness the familiar and unfamiliar about what is swimming in the seas, flying in the air, or stalking the land 500 million years from now.
Recent Comments