
One of Charles R. Knight’s paintings of Smilodon fatalis, this one menacing a giant sloth stuck in tar (off panel).
There are few fossil mammals that are as impressive the saber-toothed cat Smilodon fatalis, but despite it’s fearsome dentition some recent new reports have suggested it was more of a pussycat when it came to bite strength. This seems to be counter-intuitive; how could such an impressive animal be associated with the term “weak”? Part of it has to do with word choice, but the larger issue has to do with the fact that the bite of Smilodon wasn’t as strong as that of some other carnivores (extinct and extant), as well as dentition and feeding ecology. This issue goes far beyond just one genus or species, however, as Smilodon was only one of many genera that bore massive canines. In fact, huge “sabers” have evolved over-and-over again in the mammalian lineage (see this post and also this post for information about the cat-like ones), including the famous fangs of the machairodontine felids (saber-toothed cats) and their look-alike nimravid relatives.
Lateral, anterior, and dorsal views of the herbivore Uintatherium (Note the prominent canines). From Marsh, O.C. “The Fossil Mammals of the Order Dinocerata.” The American Naturalist, Vol. 7, No. 3. (Mar., 1873), pp. 146-153
Skull of another member of the Dinocerata; “Loxolophodon cornutus” (today known as Eobasileus cornutus). Again, note the prominent canine. From Cope, E.D. “The Amblypoda (Continued).” The American Naturalist, Vol. 19, No. 1. (Jan., 1885), pp. 40-55.
Although this post will primarily be concerned with the great “sabercats,” large, dagger-like canine teeth having been evolved multiple times by many different unrelated animals during the course of life on earth. In some herbivorous creatures, like the extinct Uintatherium and even in the extant Musk Deer, the fangs reflect sexual dimorphism and probably sexual selection, but the sharp teeth don’t seem to have a prominent function in mastication or processing of food. Likewise, large canine teeth are present in living baboons (Papio sp.), and the sexual dimorphism exhibited between the dental equipment of the males and the smaller canines of the females has long been noted (males often yawn to show off their canines, the size of their teeth being very intimidating indeed). Do the same considerations of sexual selection and dimorphism hold true for the saber-toothed cats, too? Unfortunately, fossil evidence does not always allow comparisons of the two sexes, but extant big cats and some death-trap sites have provided some information to work with. From Salesa, et al. (2006);
Among the Carnivora, sexual dimorphism is more marked in canine size than in other dental features or skull size, and these differences can be related to the breeding system. Species in which a male defends a group of females tend to be more dimorphic than those with monogamous pairs or groups of males and females. Felids are dimorphic animals, but mainly in reference to body size, with the mane of male lions being a unique example of morphological variation between sexes among the family.
This makes sense; if a male keeps a harem of females and has to defend it from other males, the species is more likely to exhibit sexual dimorphism than not. In cats, however, it seems to be more about body size (and possibly characters that wouldn’t fossilize in extinct species) than about tooth size (which would serve important other functions, so any sexual selection would be mitigated by natural selection), although we can’t be sure of this being that there are no living sabercats to study. Personally, I think there could be a sexual-selection component in some groups, but the saber-canine is so prominent in so many extinct felids and nimravids that it is extremely doubtful that all the lineages converged on similar tooth structures because of sexual selection/dimorphism, the functional advantage of larger teeth likely coming first. A lack of sexual dimorphism when considering morphology as a whole, however, may suggest a more solitary lifestyle where territories may or may not overlap are maintained and direct competition for females is not as fierce, especially since the females move through territories rather than living with a male. Such a strategy may have been employed by the late Micoene sabercat Paramachairodus ogygia. Salesa, et al. (2006), working with an assemblage made up of many of the more basal felids, have even been able to come up with a hypothesis about life history of the ancient animals based upon their finds in Spain;
[T]he probable territorial behaviour for Par. ogygia would be very similar to that of jaguars, in which males defend large, overlapping territories that include smaller territories of several females. This model is similar to that of the leopard, but in this species male territories never overlap, which could explain the different sexual dimorphism index of this species with respect to Par. ogygia and jaguar…
So, if Par. ogygia behaved more like jaguars and leopards than lions, the presence of juveniles in the trap would be highly improbable, as is the case. But in addition to the scarcity of juveniles, the sample from Batallones-1 has another interesting feature: it is mostly composed of young adults, that is, individuals with the complete permanent dentition, but without any trace of wear. These animals, which would have recently become independent of their mothers, would not as yet have had any territory, moving instead through the ranges of other adults and being more easily attracted by an easy meal, such as carrion. This age distribution therefore suggests that the sample of Par. ogygia trapped in Batallones-1 corresponds to that fraction of non-resident young individuals, both males and females, which were in a phase of dispersion. In the case of leopards, such individuals are more daring – or less cautious – than adults, and they have been seen crossing rivers in spate, whereas resident adults only cross at times of lower water. It has also been noticed that among these individuals, males are even more inclined to make these incursions than females, which remain longer with the mother, especially if there is good availability of food. If this pattern of dispersion behaviour applied to the young adults of Par. ogygia, it is likely that they were trapped in Batallones-1 more often than the resident adults.
Saber-Toothed Felid and Nimravid diversity (click for a larger image). From Emerson, S.B., and Radinsky, L. “Functional Analysis of Sabertooth Cranial Morphology.” Paleobiology, Vol. 6, No. 3. (Summer, 1980), pp. 295-312.
While the life histories of extinct mammalian carnivores are interesting in and of themselves, it is the teeth and terrifying bite of the sabercats that we are most concerned with here. Smilodon is the celebrity of saber-toothed cats, but the fossil record preserves a wide diversity of carnivores with large canine teeth, and even within the larger groupings there are even more subdivisions, the skulls of saber-toothed felids being widely variable. As discussed in the background material, nimravids are saber-tooth look-alikes that diverged from a common ancestral line earlier than the carnivores that would give rise to Smilodon, but the two lines are still closely related and have undergone parallel evolution. There is still some reshuffling of taxa going on and the true evolutionary history/affinities of many of the forms is still being worked out, but most forms you’re likely to see grouped together at a museum fall into either the nimravid or felid camps. The focus of this essay, however, will be on felids, and although they are often discussed along with their nimravid cousins the larger amount of work has been done on the felids and so we must leave the nimravids.
With the felids, then, there seem to be three kinds of sabercat that hint at differing predatory tactics, prey, and habitat. Indeed, evolution did not create carbon copies of the same creature, barring life from becoming adapted to varying circumstances; there is more variety than would be first assumed if we based all our research on the presence of prominent canines. Instead, there seem to be three “ways of being” a saber-toothed cat, as outlined by Martin, et al.;
Saber-toothed carnivores… have been divided into two groups: scimitar-toothed cats with shorter, coarsely serrated canines coupled with long legs for fast running, and dirk-toothed cats with more elongate, finely serrated canines coupled to short legs built for power rather than speed. In the Pleistocene of North America, as in Europe, the scimitar-cat was Homotherium; the North American dirk-tooth was Smilodon. We now describe a new sabercat from the Early Pleistocene of Florida [Xenosmilus], combining the scimitar-tooth canine with the short, massive limbs of a dirk-tooth predator. This presents a third way to construct a saber-toothed carnivore.
Xenosmilus hodsonae, Homotherium cf. crenatidens, and Homotherium serum. From Martin, L.D., Babiarz, J.P., Naples, V.L., and Hearst, J. “Three Ways To Be a Saber-Toothed Cat.” Naturwissenschaften, Vol. 87, No. 1 (Jan. 2000), pp. 41-44
As Martin notes, there appears to be a number of adaptational “trade offs” that sabercats in North America and Europe were subject to; fast-moving gracile forms had shorter sabers, but stouter and more powerful forms had the longer, more laterally flattened canine teeth. The “third way” that combined characters from both groups was exemplified by Xenosmilus (which Martin, et al. say would have seemed more like a bear than a cat, despite actual evolutionary relationships to the contrary). Still, leaving the overall structure of the body aside for a moment, the arrangement and sizing of the teeth of the different groups can be very telling. Martin, et al. again lay out what the usefulness of the differing tooth arrangements;
When biting, the long sabers of dirk-toothed cats may have cut parallel slits for some distance before the relatively smaller incisors could be applied. In scimitar-toothed cats the shorter canines and longer incisors worked more as a unit, first cutting parallel slits with the canines, immediately followed by the incisor arc removing the strip of flesh. Such a large open wound would have bled profusely, traumatizing the victim. If the incisors and canines acted in unison, the torsional forces on individual teeth would have been reduced, resulting in fewer restrictions on bite placement. In felids the size of the sagittal crest is directly proportional to the forces exerted by the temporalis musculature. Scimitar-toothed cats have a sagittal crest that is generally less pronounced than that in their dirk-toothed contemporaries. In a modification of the typical scimitar-tooth condition, the new cat from Florida exhibits both an elongated sagittal crest and an enlarged temporalis muscle that would have permitted a stronger bite.
While such a passage might not seem significant at first, it shows that there is more going on in a sabercat’s skull that is important to biting than just the size or shape of the canines. The placement of the incisors, for instance, seem to make a difference in biting strategy and force, dirk-toothed cats like Smilodon exhibiting a condition where the incisors are out forward of the canines. When this is taken into account, as well as the length of the canines, it seems that the canines would slash for quite some distance before the incisors could be used at all in comparison to the scimitar-toothed sabercats, the placement of the incisors in scimitar-tooths seemingly strengthening the biting teeth at the front of the jaw. The sagittal crests of these creatures should also be taken into account, such structures giving students of paleontology an indication of how carnivores (or herbivores, in the case of gorillas) have been adapted to achieve higher bite forces. Such ridges atop the skull for muscle attachment are not unique to sabercats, however, and there are some animals that have taken the structure to even greater extremes;

The extinct “bear dog” Amphicyon at the AMNH. Note the size of the sagittal crest, the reduction of the bony enclosure around the eyes, and the large holes on the side of the skull for jaw muscle attachment.

The extinct “saber-toothed” creodont Hyaenodon at the AMNH. Again, note the sagittal crest, reduction of bone enclosure around the eye, and the large canines.

The skull of the nimravid Hoplophoneus on display at the AMNH. Note the size of the canines and sagittal crest in comparison with Hyaenodon and Amphicyon.

The skull of Smilodon on display at the AMNH.

The skull of the marsupial predator Thylacoleo at the AMNH. Note the large openings on either side of the skull for the jaw muscles.

Ventral view of the skull of Thylacoleo. From E.D. Cope’s “The Tertiary Marsupialia” in The American Naturalist, Vol. 18, No. 7. (Jul., 1884), pp. 686-697.
Looking at the various groups, all show adaptations that increase the amount of available muscle attachment to achieve more powerful bites, modifying the skull in two ways. First, a sagittal crest (as already discussed) is often present to some degree, often being greater in omnivores or bone-crushing carnivores as they require greater forces to crack hard foods (although recent research by Wroe, et al. suggest that bone crushers like Spotted Hyena might not have the highest bite forces). Likewise, the holes between the skull and cheek bones are often enlarged or widened (the extreme of this group being Thylacoleo), the more muscle that can pass from lower jaw to skull being directly correlated to bite strength. What is interesting about sabercats, when considering these factors, is that they seem to be in the middle. They don’t exhibit adaptations of the skull to the extreme as in Amphicyon or Thylacoleo, but they still exhibit changes allowing for powerful bites (strong enough to kill and consume prey, at least). The trend is obvious and has not been missed by reseachers, and Emerson says the following about it;
With enlargement of upper canines, skulls of paleofelid, neofelid, marsupial and, as far as the record shows, creodont sabertooths were remodeled in similar ways. This evolutionary convergence in cranial morphology is not surprising, since most of the modifications relate to allowing increased gape while retaining bite strength at the carnassial. Those are factors essential for all sabertooths, and the possible ways to achieve them, starting from a generalized mammalian cranial morphology, are limited…
Why did sabertooth specializations evolve so many times? Their multiple evolution, plus the fact that several species of sabertoothed felids existed for most of the history of the family (from about 35 Myr to about 15,000 yr BP) suggest that sabertooth canines provided an effective alternative to the modern carnivore mode of killing prey

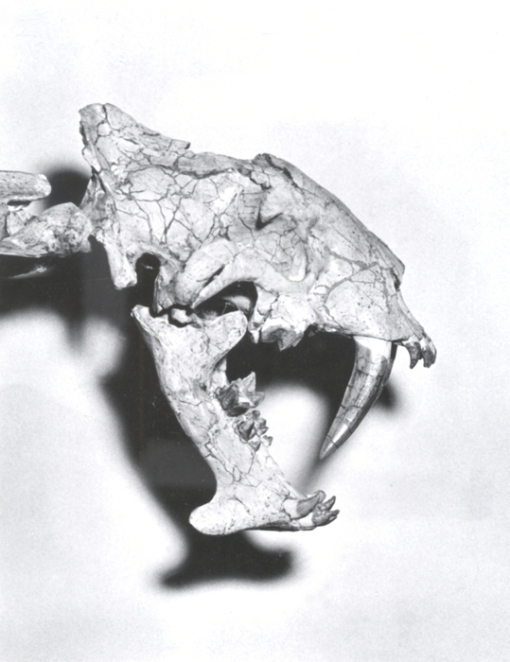
The skull of the saber-toothed cat Megantereon. Like in Smilodon, not how the incisors jut out (as well as the overly large nasal opening in this genus).
The basic mechanics of the skull just discussed gives researchers clues as to how sabercats could have killed their prey, but reconstructing ancient predator/prey interactions with no exact modern equivalent is difficult. Indeed, debate has gone on for years as to how sabercats used their teeth to bring down prey (see Simpson’s paper), either by stabbing, cutting, slicing, or even (as silly as it may seem) by crushing. What does seem apparent today, however, is that the canines of the sabercats were relatively delicate, and it would be unwise to fully sink them into a struggling animal as they may easily be broken off. Even if such an attempt to deeply puncture a prey item was not undertaken, biting full-force into bone could have also easily damaged teeth (or even broken them off), making it unlikely that sabercats jumped onto the back of their prey and tried to sink their teeth into the back of the prey’s skull like some modern cats. Recent research has even shown that the skull of Smilodon was ill-suited to handle stresses associated with struggling prey when compared to the skull of a lion, and I wonder how often individual Smilodon perished because of stresses associated with taking down prey if the victim was not brought down and killed quickly. Indeed, it seems that the long teeth were better suited to slicing soft flesh, i.e. cutting open the belly of prey or slicing open the throat, rather than piercing rough hides and ramming through bone.
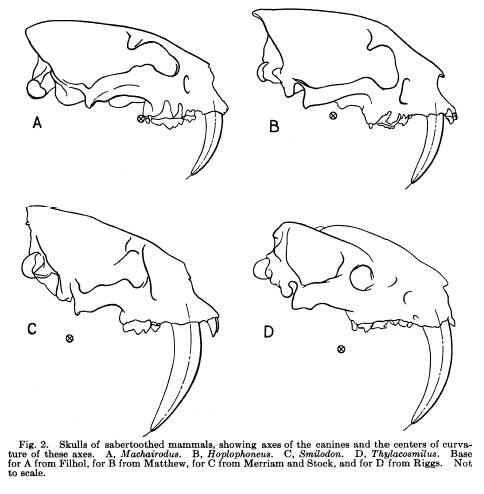
Skulls (mandibles not pictured) of 4 “saber-toothed” mammals from “The Function of Saber-Like Canines in Carnivorous Mammals” by G.G. Simpson, American Museum Novitiates, August 4, 1941. Pictured are A) Machairodus (felid), B) Hoplophoneus (nimravid), C) Smilodon (felid), and D) Thylacosmilus (marsupial).
As just discussed in terms of tooth and skull stressed, many factors of life history, behavior, and morphology of extant big cats and sabercats might be similar, but the massive canines of the extinct group seem to infer a different killing strategy, and there is no reason to assume that they were like modern big cats in every respect. Salesa, et al. sums it up this way;
Extant felids kill small animals by biting on the nape or directly on the skull, using their rounded-section canines, but if any sabre-toothed cat tried to do this they would have risked breaking the laterally flattened upper canines. For this reason, it is more probable that they developed some behavioural mechanism to minimize that risk, such as ignoring prey below a given size. It is likely that machairodontines developed this ethological trait early in their evolution, and so narrowed their prey size range in comparison with that of felines, which hunt both large and small animals. This high specialization has been pointed out as one of the possible reasons for the gradual decline and final extinction of the sabre-toothed cats in the Pleistocene… The development of this strategy was probably the key reason for the sabre-tooted cats becoming the dominant predators in the land mammal faunas from the Late Miocene to Late Pleistocene.
It might not immediately make sense that felids with fragile teeth would specialize in eating large prey, but that is whale the fossil evidence (as we currently understand it) infers. While the smallest prey would pose no problems (outside of not being a fully satisfying meal), but medium sized prey with smaller areas of soft flesh (like the stomach and neck) would potentially be more dangerous and a more exact bite would be needed to prevent damage to the teeth and skull. Hence, it seems that the slashing and ripping of soft tissue in larger animals was the main method of killing prey (after it had been brought down or slowed by blood loss), taking hypercarnivory to an even more specialized extent.

An Amur Leopard yawns. Note the relatively small (but still fearsome) canines of the upper and lower jaw.
What, then, of a smaller living cat, the Clouded Leopard (Neofelis nebulosa and N. diardii), which has been heralded as a modern analog of sabercats? As Christiansen notes, Clouded Leopards are a bit bizarre, and it is incorrect to call them “small” big cats or modern sabercats, the genus showing a number of convergences with extinct forms while remaining distinct from the famed genus Panthera;
The skull morphology of the clouded leopard sets it apart from other extant felids, and in a number of respects it approaches the morphology of primitive sabertooths. This indicates convergence of several characters in machairodontine felids and the clouded leopard, mainly as adaptations for attaining a large gape. This raises doubts about the characters hitherto considered as distinguishing sabertoothed from nonsabertoothed predators…
Clearly, Neofelis and the sabertooths independently evolved a suite of the same specializations for the same overall purpose of attaining a large gape, a prerequisite for efficient jaw mechanics with large canines, but the reasons for evolving these characters need not have been similar. Based on analyses of lower jaw bending moments and inferred resistance to mechanical loadings, Therrien (2005) suggested that Neofelis could be at the beginning of a new sabertooth radiation. Such claims are difficult to test, however, since the extant sister taxon to Neofelis (Panthera) shares none of its sabertoothed characters, and the fossil record provides no clues of felids closer to Neofelis than Panthera. At present, however, there is little evidence to suggest that Neofelis can be regarded as an “extant sabertooth,” although it clearly shares a number of characters with them that are absent in other extant felids. On the other hand, it cannot be regarded as simply an intermediate between large and small felids, as normally assumed. The presence to some extent of characters normally ascribed to sabertooths in Neofelis raises doubts about their functional and evolutionary significance in primitive machairodonts such as Nimravides or Paramachairodus, hitherto the only reasonably well-known primitive machairodont. Such animals need not have shared the presumed functional skull morphology of later, more derived sabertooths and are perhaps not to be regarded as “sabertoothed” at all, if by sabertoothed is implied animals functionally significantly different from extant felids.
Again, this shows a convergence of functional morphology despite existing evolutionary relationships, many felids being adapted in similar ways. As stated previously, the large canines of saber-toothed predators required the animals to open their jaws wide but also narrowed their predatory niche to some extent. Likewise, various tests seem to show that the bite of sabercats like Smilodon was “weak,” with news reports often relating that the terrible felids were more like big housecats when compared to living big cats. This is a mistake (and it would be a grave one for anyone ever to cross a sabercat), born of a lack of recognition that bite forces exist on a continuum and are related to a number of factors and cannot simply be deemed “weak” or “strong” without further comment. Christiansen relates the bite force of Smilodon as such;
[A]lthough large sabertooths such as Smilodon and Homotherium had weaker bite forces than lions or tigers, their bite forces were broadly comparable to those of jaguars and large leopards, and, thus, cannot be claimed to have been “weak”. Lower bite forces at any given body size were probably evolutionarily possible owing to a marked contribution from the upper cervical musculature to the killing bite, which… was absent in Neofelis and primitive machairodonts such as Paramachairodus. Thus, bite force analysis may constitute a hitherto overlooked parameter in evaluating whether or not primitive machairodonts such as Paramachairodus or Nimravides really did employ a canine shear bite with a marked contribution from the cervical muscles to subdue prey, or killed in a manner similar to extant felids, which requires a stronger killing bite…
In many Plio-Pleistocene communities predator competition was more severe than today, and a sabertooth killing mode could be a way of ensuring faster kill rates, since a throat shear-bite most likely would kill prey faster than a throttling throat bite, common in extant pantherines. In lions, it can take up to 13 minutes to kill large prey, and in such cases the prey is frequently killed by disemboweling by other pride members. In the cheetah a suffocation bite can take even longer to kill prey. Carcass theft and feeding competition is very common among extant large, sympatric predators, and a faster kill mode could be a way of reducing the risk of carcass theft from competing predators. In many large predators with sympatric competitors, rapid consumption can be a way of reducing the risk of carcass theft, and this would most likely have been accentuated in past ecosystems with more intense large predator competition. Accordingly, the morphology and behavior of extant predators need not reflect the circumstances to which they became adapted when they evolved. More intense competition could accelerate the evolution of a sabertooth morphology…
This passage reflects the problems with reconstructing bite forces and predation techniques of extinct creatures; more is involved than just the opening and closing of the jaw. The neck muscles of many sabercats (except in some of the more basal members, as noted) likely contributed to the strength of the bite in a way that’s not directly testable today. Likewise, the killing technique of sabercats might not have required a bite as strong as a modern-day tiger, as in a land filled with other predators, it might simply take too long to try and suffocate a prey animal or bite through the back of their skull. Disemboweling or tearing out the throat of the prey item, by contrast, is a much quicker way to do large amounts of damage but it seems that it would require teamwork, solitary extant big cats often opting for a killing neck bite when the prey has been brought down. Even if this is eventually shown to be incorrect, it should be remembered that bite strength is not everything; despite its large size, the Great White Shark (Carcharadon carcharias) has a relatively weak bite, but it makes up for it with heavily serrated teeth, force of impact when attacking prey, and side-to-side head shaking to saw through its food. Crocodilians, by contrast, have very strong bite forces but they don’t saw through prey or chew, the emphasis being holding on to struggling prey and drowning it before ripping it apart. Such considerations bring us to another point mentioned above in our discussion of scimitar-tooths vs. dirk tooths in that the famous dirk-toothed cats like Smilodon were more powerfully built, seemingly focusing on bringing a large animal down to the ground and then delivering devastating bites once the stomach and neck were exposed (a process that would be made easier by groups working together, as seen in modern examples like lions bringing down giraffes or elephants).
A group of lions brings down a giraffe.
A group of lions brings down an elephant.
A new paper, just out in PNAS, does take the powerful neck muscles of Smilodon into account, however, and the information from the new models appear to corraborate the modern understanding of a felid that captured and killed prey in a way quite different from Panthera. From McHenry, et al.;
Our results demonstrate that bite force driven by jaw muscles was relatively weak in S. fatalis, one-third that of a lion (Panthera leo) of comparable size, and its skull was poorly optimized to resist the extrinsic loadings generated by struggling prey. Its skull is better optimized for bites on restrained prey where the bite is augmented by force from the cervical musculature. We conclude that prey were brought to ground and restrained before a killing bite, driven in large part by powerful cervical musculature. Because large prey is easier to restrain if its head is secured, the killing bite was most likely directed to the neck. We suggest that the more powerful jaw muscles of P. leo may be required for extended, asphyxiating bites and that the relatively low bite forces in S. fatalis might reflect its ability to kill large prey more quickly, avoiding the need for prolonged bites.
Hunting isn’t the only aspect of sabercat predation that seems to have differed from modern carnivores; they way they ate (and what they ate) is somewhat at variance with modern forms, as well. As is apparent at this point, the contact of the canines with bones would have been avoided, and it seems that the hard parts of the skeleton would have been avoided when a sabercat was consuming it. This could differ among different groups (perhaps some of the shorter-toothed forms not being so finicky about bone), but research into microwear patterns on teeth of Smilodon don’t seem to match with wear patterns of any living carnivores, suggesting a different dietary preference. It could be hypothesized, then, that creatures like Smilodon primarily consumed the soft parts of the carcass or what could be removed without too much damage to the teeth, and it should be remembered that living big cats often do not eat every part of the skeleton. Some, like cougars, have favored parts that they eat but end up leaving as much as 40% of the carcass behind. Other predators, especially bone-crushing ones, could take advantage of the leftovers, although the felids might have had to eat quickly as some of their osteophagus competitors may not have been patient (and, in fact, lions and hyenas often fight over kills and steal them from each other today).
Given all the prior considerations, it now seems that sabercats specialized in bringing down relatively large prey down quickly (some likely working in groups to do so), killing the victims by slashing open their stomachs or slicing through the blood vessels of the neck. This would be a much messier, but quicker, method than employed by living big cats, although the limitation of food sources likely caused in the eventual downfall of sabercats. Hypercarnivory can be a dangerous adaptive path to go down, and cats are clearly the most meat-dependant of the Carnivora, but it seems that extinct forms took their dental and dietary specialization above and beyond what is seen today. The price paid for such adaptations ended up being extinction, but given how many times they have shown up in the history of life on this planet, someday there may again be a saber-toothed predator stalking the shadows.
References;
Anyonge, W. “Microwear on Canines and Killing Behavior in Large Carnivores: Saber Function in Smilodon fatalis” Journal of Mammalogy, Vol. 77, No. 4 (Nov., 1996), pp. 1059-1067
Christiansen, P. “Canine morphology in the larger Felidae: implications for feeding ecology.” Biological Journal of the Linnean Society. Vol. 91, No. 4 (Aug., 2007), pp. 573-592
Christiansen, P. “Sabertooth characters in the clouded leopard (Neofelis nebulosa Griffiths 1821).” Journal of Morphology, Vol. 267, No. 10 (Jul., 2006), pp. 1186 – 1198
Christiansen, P. and Wroe, S. “Bite Forces and Evolutionary Adaptations to Feeding Ecology in Carnivores.” Ecology, Vol. 88, No. 2 (Feb., 2007), pp. 347–358
Cope, E.D. “The Amblypoda (Continued).” The American Naturalist, Vol. 19, No. 1. (Jan., 1885), pp. 40-55.
Cope, E.D. “The Tertiary Marsupialia.” The American Naturalist, Vol. 18, No. 7. (Jul., 1884), pp. 686-697.
Emerson, S.B., and Radinsky, L. “Functional Analysis of Sabertooth Cranial Morphology.” Paleobiology, Vol. 6, No. 3. (Summer, 1980), pp. 295-312.
Leutenegger, W., and Kelly, J.T. “Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates.” Primates, Vol. 18, No. 1 (Jan., 1977), pp. 117-136
Lucas, P.W., Corlett, R.T., and Luke, D.A. “Sexual dimorphism of tooth size in anthropoids.” Human Evolution Vol. 1, No. 1 (Feb., 1986), pp. 23-39
Marsh, O.C. “The Fossil Mammals of the Order Dinocerata.” The American Naturalist, Vol. 7, No. 3. (Mar., 1873), pp. 146-153
Martin, L.D., Babiarz, J.P., Naples, V.L., and Hearst, J. “Three Ways To Be a Saber-Toothed Cat.” Naturwissenschaften, Vol. 87, No. 1 (Jan. 2000), pp. 41-44
McHenry, C.R., et al. “Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation.” PNAS, Published online before print October 2, 2007
Salesa, M.J., et al. “Aspects of the functional morphology in the cranial and cervical skeleton of the sabre-toothed cat Paramachairodus ogygia (Kaup, 1832) (Felidae, Machairodontinae) from the Late Miocene of Spain: implications for the origins of the machairodont killing bite.” Zoological Journal of the Linnean Society, Vol. 144, No. 3, (Jul., 2005) pp. 363-377
Salesa, M.J., et al. “Inferred behaviour and ecology of the primitive sabre-toothed cat Paramachairodus ogygia (Felidae, Machairodontinae) from the Late Miocene of Spain” Journal of Zoology, Vol. 268, No. 3 (Mar., 2006), pp. 243-254
Simpson, G.G. “The Function of Saber-Like Canines in Carnivorous Mammals.” American Museum Novitiates, August 4, 1941
Therrian, F. “Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators.” Journal of Zoology, Vol. 276, No. 3 (Nov., 2005), pp. 249-270
Therrian, F. “Feeding behaviour and bite force of sabretoothed predators.” Zoological Journal of the Linnean Society, Vol. 145, No. 3 (Nov., 2005), pp. 393-426
Van Valkenburgh, B., and Molnar, R.E. “Dinosaurian and mammalian predators compared.” Paleobiology, Vol. 28, No. 4 (Dec., 2002), pp. 527–543
Walker, Alan. “Mechanisms of honing in the male baboon canine.” American Journal of Physical Anthropology, Vol. 65, No. 1 (?, 1984), pp. 47 – 60
Wroe, S., McHenry, C., and Thomason, Jeffery. “Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa.” Proceedings of the Royal Society B, Vol. 272, No. 1563 (Mar., 2005), pp. 619-625







































Recent Comments