Note: Welcome, visitors from The Sandwalk and Pharyngula! I am certainly humbled by the amount of praise and attention this post has received, and although it’s not as scientifically rigorous as I would have liked to be (I still have much to learn), I hope that you find it to be an enjoyable read all the same.
Update: I’ve created something of an appendix to this article about how creationists have presented horse evolution in some of their books. It can be found here.

One of Charles R. Knight’s interpretations of Eohippus
When the name of O.C. Marsh is invoked, it is often to tell of his participation in the great “Bone Wars” of the late 19th century, sparring with fellow osteophile E.D. Cope in the pages of the New York Herald. Twisted tales of deceit and sabotage were promulgated in the sensationalist paper, and while both men helped to bring about an American revolution in vertebrate paleontology, the scars of their bitter squabbling have yet to fully heal. Such scientific in-fighting might seem worthy only of a historical footnote or an introduction to the stereotyped image of “smash-and-grab” paleontology of the time which is almost romantically referred to, but the truth of the matter goes far deeper than the public beard-pulling that is so often remembered.
The tiff between Cope and Marsh is strange in that is seems to exist in the popular literature out of time, removed from the context in which it had originally existed. Charles Darwin had published his earth-shaking work On the Origin of Species by Natural Selection a scant 31 years before the ink almost ran red with rage on the pages of the Herald, the question of evolution being of far more importance in the public consciousness than dinosaurs. The full establishment of the dinosaur as a cultural (and dare I say, mythical) creature in the mind of the American public only seemed to take place after the Bone Wars, the appointment of Henry Fairfield Osborn to the American Museum of Natural History (specifically hired to establish a vertebrate paleontology program) and the popular reports of the dinosaur that carried the namesake of Andrew Carnegie, Diplodocus carnegei, being the more immediate beginnings of the public’s love affair with the extinct creatures. Before Brontosaurus and Tyrannosaurus became household names, the public eye was focused upon horses and birds.
The latter half of the 19th century was a stirring time for biological science, especially involving the new areas of vertebrate paleontology and evolution, the august authorities in England keeping on eye on the up-and-comers starting their own careers in the states. Early on, paleontologist E.D. Cope impressed T.H. Huxley with his 1866 discovery of Laelaps aquilunguis, but in a paleontological clean-sweep Marsh would eventually have his name attached to Cope’s dinosaur and the admiration of not only T.H. Huxley, but Charles Darwin himself. As for the renaming of Laelaps, Marsh found that the name was already taken by a genus of mite, renaming the New Jersey greensand dinosaur Dryptosaurus in 1877 (although Cope, throughout the rest of his career, called the dinosaur Laelaps). It would take more than some taxonomic shuffling to impress the eminent British anatomists and paleontologists, however, and Marsh’s ticket into Huxley’s good graces came in the form of toothed Cretaceous birds like Hesperornis (Marsh, 1872).
While the discovery of ancient bones was exciting to some, evolution was an even more popular topic, and the question that surrounded every fossil was “How does this fit into the grand scheme of evolution?” The 1861 discovery of Archaeopteryx from the lagerstatten of Solnhofen, Germany seemed to arrive right on cue to confirm that evolution had taken place in times previously referred to as “antediluvial”, and Marsh’s subsequent discovery of birds with teeth in the American West further confirmed the notion that aves had evolved from reptilian ancestors (Huxley even being so progressive as to name the dinosaurs as the probable ancestral stock). Charles Darwin himself recognized the importance of Marsh’s discoveries as well, and two years after Marsh visited Darwin at Down House in 1878, Darwin wrote the following letter to Marsh on or about August 31, 1880;
I received some time ago your very kind note of July 28th, & yesterday the magnificent volume. I have looked with renewed admiration at the plates, & will soon read the text. Your work on these old birds & on the many fossil animals of N. America has afforded the best support to the theory of evolution, which has appeared within the last 20 years. The general appearance of the copy which you have sent me is worthy of its contents, and I can say nothing stronger than this.
With cordial thanks, believe me yours very sincerely,
Charles Darwin
Toothed birds were not Marsh’s only claim to evolutionary fame, however; by 1876 his assistants had collected enough fossil horse material to show that the horse was not “a gift from the Old World to the New” (as an European authority had once said during a lecture), but quite the reverse. In fact, the timing of the discovery and study of the horses could not have been better for Marsh, as in 1876 T.H. Huxley visited Yale and was duly impressed with the American Professor and his fossil horses. Huxley was absolutely enthralled by Marsh’s fossil equids, and Huxley’s son Leonard wrote of the visitation upon the New World horses as follows;
At each inquiry, whether he had a specimen to illustrate such and such a point or exemplify a transition from earlier and less specialized forms to later and more specialized ones, Professor Marsh would simply turn to his assistant and bid him fetch box number so and so, until Huxley turned upon him and said ‘I believe you are a magician; whatever I want, you just conjure up.'”
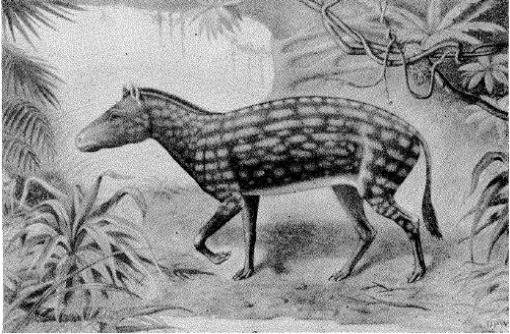
Restoration of Eohippus. From “The Dawn Horse or Eohippus” by Chester Stock (1947).
Huxley even featured Marsh’s discoveries of toothed birds and fossil horses in a set of three lectures he delivered at Chickering Hall in New York, the visit of such a famous evolutionist being front page news (with Marsh sharing in good press since his fossils were discussed by so prominent a figure as Huxley). The only thing that could have made the event sweeter would have been the knowledge of an ancestral horse with five toes (what was regarded as the “primitive” condition for mammals as far as digits go), and Huxley prophesied that such a creature would likely be found in North America. In truth, while it did not precisely fit the bill, a horse bearing a vestigial fifth toe had already been found and was collecting dust in Marsh’s Peabody museum. Writing to Huxley on July 12, 1877, Marsh revealed that little Eohippus (a name that was given up when it was discovered that Richard Owen’s Hyracotherium had priority, only to be later changed back to Eohippus in recent years) had been right under his nose all along;
I had him “corralled” in the basement of our Museum when you were there, but he was so covered with Eocene mud, I did not know him from Orohippus. I promise you his grandfather in time for your next horse lecture if you will give me proper notice.
Although the popular press did not take much note of the re-discovery of Eohippus, Huxley was well pleased, and promised to show Marsh all the “lions” of British science during his aforementioned 1878 visit. Such close ties would be important to Marsh later on, serving to keep Cope out of some respectable circles as well as giving Marsh a good amount of prestige. Oddly enough, however, Cope had his own horse genealogy (although ignoring Marsh’s labels) that went from four toes to one, and it was Cope’s “dawn horse” that provided the basis for some of the first Eohippus reconstructions, not Marsh’s. While Cope missed out on a golden opportunity in 1872 when he was provided a jaw fragment of an early horse, Marsh’s skeleton (as far as I can tell) remained locked away while Cope’s employee J.L. Wortmann uncovered the rest of Cope’s specimen of Eohippus in 1880. Cope named his animal Protorohippus, and it was his reconstruction that ultimately influenced Charles R. Knight and, subsequently, Rudolph Zallinger when he painted his famous Age of Mammals mural. For those who did not get at least a chuckle out of the last line, Zallinger created his mammalian masterpiece for the Peabody museum, the very establishment that O.C. Marsh had created to start his professional career.
As can be said of any scientist, however, Cope and Marsh were both products of their time and (especially in their respective cases) their egos, and while the fact that horses evolved was proved beyond doubt, both men made mistakes when it came to evolution. While Cope, late in his career, bemoaned the fact that Marsh had poisoned the well when it came to making connections with Huxley and other British scientists, it is doubtful that Cope would have lasted long amongst those of the Darwinian school of evolution. In the 1896 book The Primary Factors of Organic Evolution, Cope saw the evolution of the horse being orthogenic, or proceeding in such a way as to imply direction towards a more adapted or perfected form. As this concerns horses, Cope wrote;
Examination of all these genealogical lines reveals a certain definiteness of end and directness of approach. We discover no accessions of characters which are afterwards lost, as would naturally occur as a result of undirected variation. Nor do we discover anything like the appearance of sports along the line, the word sport being used in the sense of a variation widely divergent from its immediate ancestor. On the contrary, the more thorough becomes our knowledge of the series, the more evident does it become that progressive evolution has advanced by minute increments along a definite line, and that variations off this line have not exerted an appreciable influence on the result.
Such notions would have gotten Cope banned from Finch’s Beak gentleman’s association (if one had actually existed), the concept of directed evolution undermining one of the most important points that Darwin had attempted to make about the “transmutation” of life on earth. As we shall later see, however, such notions of orthogenesis may have had some influence on one of Cope’s latter-day pupils, Henry Fairfield Osborn, as well.

An illustration of the horse “Clique,” which had an extra toe on each fore-foot. Marsh examined this horse while still alive, and the horse was donated to Yale after its death in 1891. From Marsh, O.C. 1892. “Recent Polydactyl Horses.”
Marsh, as has already been determined, definitely had the attention of the progenitors of evolution by natural selection, and through the efforts of Matt Wedel, Randy Irmis, and Mike Taylor a number of Marsh’s writings have become available for viewing on the internet (The Marsh Repository). In a 1879 paper published some time after Huxley’s visit, “Polydactyle Horses, Recent and Extinct,” Marsh prefaces the rather short fossil section with several pages about known horses within recent history that had extra digits. The most typical condition for the differing equines was having an extra digit on the inside of the front hooves, one that did not touch the ground. Coupled with a brief appeal to similar observations of extra toes from development, this approach was indeed a wise one; not only do most living horses have vestiges of digits that have been lost, sometimes a multi-toed condition still occurs in living animals, seemingly fitting with the same trends seen in extinct genera.

O.C. Marsh’s concept of “The Geneology of the Horse,” a decidedly straight-line progression. From Marsh, O.C. 1879. “Polydactyly Horses, Recent and Extinct.”
What is notable about Marsh’s interpretation of the history of horse evolution is how straightforward it is. Although missing Cope’s differing evolutionary hypotheses, Marsh makes no qualifications about the fossils he found representing only the “types” of different horses; horses evolved along a straight line, and while a few steps may be missing, it was not indicative of the widely branching pattern recognized by later scientists. The “extraneous” toes seem to become reduced in a gradual fashion, while size and tooth height increased (although the patterns on the teeth, as can be seen in the illustration, vary quite a bit in the “higher” forms). Perhaps Marsh’s adherence to a strict linear progression was at least partly inspired by the diagnosis of Huxley. In an obituary written by Marsh to commemorate Huxley’s life, Marsh made special mention of his horses;
One of Huxley’s lectures in New York was to he on the genealogy of the horse, a subject which he had already written about, based entirely upon European specimens. My own explorations had led me to conclusions quite different from his, and my specimens seemed to me to prove conclusively that the horse originated in the New World and not in the Old, and that its genealogy must be worked out here. With some hesitation, I laid the whole matter frankly before Huxley, and he spent nearly two days going over my specimens with me, and testing each point I made. He then informed me that all this was new to him, and that my facts demonstrated the evolution of the horse beyond question, and for the first time indicated the direct line of descent of an existing animal.
Such interpretations of evolution and the fossil record could only exist within a certain paleontological framework; the more bones that were found from different times and locales the more the old notions would splinter and crack. Vertebrate paleontologists who would succeed Cope and Marsh could not study what they did not have, however, but they still recognized the importance of the horse in showing evolution to be a reality. In 1891 Henry Fairfield Osborn, an independently wealthy Princeton professor and one of E.D. Cope’s friends and supporters during the embroiled Herald fiasco, was appointed the first curator of vertebrate paleontology at the American Museum of Natural History in New York City. The museum was somewhat embarrassed at not possessing any sizable collection of vertebrate fossil material, and even though Cope eventually sold some of his collection to the AMNH for a sum that disappointed the beleaguered Philadelphian, the halls of the great institution were still found wanting of ancient creatures that would bring it notoriety.
Osborn, despite his off-kilter ideas about human evolution that plagued his later years, largely made the AMNH what it is today, having some of the best and brightest collectors and preparators of the 20th century under his employ. While such gems as Barnum Brown’s two Tyrannosaurus rex skeletons, the specimen that remains on display today being Brown’s self-confessed “favorite child,” definitely helped to make the museum famous, some of Osborn’s favorite subjects were the fossil horses. Early on in his career, Osborn attempted to raise $10,000 from museum trustees for a project involving horse evolution, but the appeal was denied. Osborn kept at it and eventually succeeded, however, securing $15,000 from William C. Whitney in 1897, funds used to send collectors and curators like James W. Gidley, Bill Thomson, W.D. Matthew, and Walter Granger out into the field to collect ever more horses from Texas, South Dakota, Colorado, and other locales. Indeed, Osborn soon had many new horse fossils to study and display, creating one of the most notable (and among Biblical fundamentalists, controversial) displays of evolution ever presented to the public.
*(WWII caused the museum to send the first, more incomplete Tyrannosaurus rex skeleton, to the Carnegie Museum out of fear that the museum would be bombed and both would be lost. This may seem like an ill-founded fear, but many fossils like Spinosaurus were lost because German museums were struck with Allied payloads.)
Osborn did much to enhance the AMNH collections during the close of the 19th century, although his rather strange views about mammalian evolution (fueled in part by racism and part by Osborn’s membership in the Presbyterian church) never found wide acceptance. Despite his pet hypotheses, Osborn sent paleontologists far and wide in search of specimens to confirm his ideas, and at least in the case of the Roy Chapman Andrews expeditions during the early 1920’s, unexpected boons abounded. While Marsh held that he had moved horse ancestry out of the Old World and safely into America’s domain, Osborn saw the origin of major placental mammalian groups stemming from Asia (including the origin of humans), the hypothetical five-toed ancestor of the horse remaining elusive in North America because it was “really” buried somewhere in Asia. Osborn described his hypothesis as follows;
In the dispersal center, during the Age of Reptiles and the beginning of the Age of Mammals, there evolved the most remote ancestors of all the higher kinds of mammalian life which exist today, including, for example, the five-toed horses, which have not yet been discovered in either Europe or America. That the very earliest horses known in either Europe or America were four-toed indicates that their ancestors may have lost their fifth toe while still resident in the Asiatic homeland.
Roy Chapman Andrews did not bring Osborn any Asiatic five-toed horses from the expeditions into Mongolia in the early 1920’s, although the mammals Paraceratherium and Andrewsarchus were exciting enough in and of themselves. .
The lack of the most ancestral mammalian fossils did not stop Obsorn from attempting to further his own hypotheses, however, and in order to understand how straight-line evolution may have been presented at the AMNH we need to know how Osborn obfuscated the role of “chance” in evolution (using it almost in the same context as modern creationists do), calling the idea that natural selection works on random variations a “dogma.” Osborn instead preferred an Aristotelian “law,” quoting the philosopher in his 1917 book The Origin and Evolution of Life;
So far as law is concerned, we observe that the evolution of life forms is like that of the stars: their origin and evolution as revealed through palaeontology go to prove that Aristotle was essentially right when he said that “Nature produces those things which, being continually moved by a certain principle contained in themselves, arrive at a certain end.”
Such a notion could be regarded as the “Restless Gene” hypothesis, with what Osborn then referred to as the “hereditary-chromatin” in the animal filling needs as they arose in order to achieve a particular end. Despite his confusion about the role of “law” and “chance” in nature, Osborn did recognize that there were certain ratios in limb structures that were present in animals filling different ecological niches, even closely related ones. In the same book, Osborn writes the following about early horses;
No form of sudden change of character (saltation, mutation of de Vries) or of the chance theory of evolution accounts for such precise steps in mechanical adjustment [as in the limb structure of horses]; because for all the proportional changes, which make up ninety-five percent of mammalian evolution, we must seek a similar cause, namely, the cause of acceleration, balance or persistence, and retardation. This cause may prove to be in the nature of physiochemical interactions regulated by selection. The great importance of selection in the evolution of proportion is demonstrated by the universal law that the limb proportions of mammals are closely adjusted to provide for escape from enemies at each stage in development.
This chain of reasoning, such as it is, nearly works backwards from evolution’s “products” (which it is never done fiddling with), much like the lampoon (which I believe stems from Voltaire, although I have been unable to find the quote) that the nose was placed on the human face to hold up ones glasses.

Assemblage of bones, illustrated as discovery in situ, of the Pleistocene horse Equus scotti. From Gidley, James Williams. 1900 “A new species of Pleistocene horse from the Staked Plains of Texas“. Bulletin of the AMNH ; v. 13, article 13.

A mounted skeleton of Equus scotti at the AMNH, constructed out of two skeletons. From Gidley, James Williams. 1901. “Tooth characters and revision of the North American species of the genus Equus.” Bulletin of the AMNH ; v. 14, article 9.
Even though Osborn’s ideas of evolution did not catch on, the idea of horse evolution as a more-or-less straight line was still a popular one, at least in works and representations meant for public consumption. The diversity of fossil horses, thanks to many of the expeditions undertaken by Osborn’s department at the beginning of the 20th century, had considerably expanded, and the idea of an evolutionary “bush” for horses began to take root. Such a representation can be seen in one such generalized and “primitive” bush provided by J.W. Gidley in a 1907 paper on horses from the Miocene and Pliocene of North America. Indeed, the diversity of three-toed forms suggested that ancestry was perhaps more complicated than previously thought, more than one form of horse existing at any one time depending on the available habitats. Osborn noted this in his 1917 book as well, but it seemed to be only a supplementary bit of information behind his ideas of a biogenetic law. One of Osborn’s hires, J.W. Gidley, had a more accurate view of horse evolution, however, and produced the first known branching phylogeny of horses through the Miocene and Pliocene.

A hypothesis as to the relationships of horse subfamilies by J.W. Gidley in 1907. This is the first known branching diagram for horse evolution. From Gidley, James Williams. 1907. “Revision of the Miocene and Pliocene Equidae of North America.” Bulletin of the AMNH ; v. 23, article 35.
As can be seen from Marsh’s earlier phyletic progression, much of horse evolution seemed to be dictated by features of teeth, the number of toes, and certain aspects of the skull, but as Gidley notes in his paper more material is needed if we are to truly understand the relationships of horses. Indeed, things were not so clean and neat as implied by Marsh’s illustration, even with the inclusion of new taxa. In the summary of the research, Gidley concludes;
As at present understood, the fact seems to be fairly well established that there is a considerable phyletic hiatus between the groups of the Equidae as above subdivided, which are as yet not bridged over by intermediate forms. Such a hiatus seems especially marked between the Anchitheriinae and the Protohippinae, while these groups greatly overlap each other in time. So far as indicated by any known species the Anchitheriinae could not well have stood in direct ancestral line to the latter group or to the Equiinae. There seems also to be almost as decided a gap between the Anchitheriinae and the known species of the older group, the Hyracotheriinie. The Equiinae may well have been derived from some species of the Protohippus division of the Protohippinae.
Outside of engaging in a more detailed study, Gidley also made note that various genera of horses overlapped in time with each other. While this does not rule out anagenesis entirely, it is a problem if there is such a large diversity of horses with similar features living alongside each other rather than a few isolated populations moving in a straight-line progression. The overlap was recognized and illustrated by W.D. Matthew almost 20 years after Gidley’s paper, showing where and when fossil horses existed;

Visual representation of the geological span and geographical ranges of equids through the Cenozoic. Such a representation could easily be misunderstood as endorsing straight-line evolution of horses. From Matthew, W.D. 1926. “The Evolution of the Horse: A Record and Its Interpretation” The Quarterly Review of Biology, Vol. 1, No. 2., pp. 139-185.
While the illustration, if followed closely, does show a branching pattern of evolution, to an untrained eye the evolution of horses through time seems to go in a relatively straight line, the overlap seemingly giving way to an almost orthogenic trend. I doubt that Matthew’s article was regular Sunday night reading for families of the late 1920’s and so I doubt that it contributed directly to mistaken notions of horse evolution, but another illustration from the same paper could more easily cause confusion;

A simplified, “straight-line” version of horse evolution (Click the image for a larger version). This figure was also reproduced in George McCready Price’s The Predicament of Evolution. From Matthew, W.D. 1926. “The Evolution of the Horse: A Record and Its Interpretation” The Quarterly Review of Biology, Vol. 1, No. 2., pp. 139-185.
This model is similar to Marsh’s (see above) in that horses seem to have followed a very simple ancestor/descendant progression through time. While it is true that living horses did have ancestors with multiple toes and we could trace their line backwards through time to the exclusion of other closely related genera, diagrams like this one seem to have “won out” in the public mind over those that more fully encompassed horse diversity. A 1940 paper by R.A. Stirton would be much clearer when it came to the branching horse lineage;

From Stirton, R. A. 1940. Phylogeny of North American Equidae. Bull. Dept. Geol. Sci., Univ. California 25(4): 165-198.
Stirton’s illustration is interesting as it shows a fairly straightforward line of descent through Miohippus is the Upper Oligocene, Miohippus giving rise to some side branches that would eventually go extinct before modern times. The radiation of the ancestors and close relatives of modern horses did not start, according to the phylogeny, until the Upper Miocene and Merychippus, Pliohippus eventually giving rise to Equus in the Upper Pliocene. Further, it is interesting to see how close Stirton’s phylogeny is to the work of later researchers, especially that fossil horse authority Bruce McFadden;

From MacFadden, Bruce. 1985. “Patterns of Phylogeny and Rates of Evolution in Fossil Horses: Hipparions from the Miocene and Pliocene of North America” Paleobiology, Vol. 11, No. 3. (Summer, 1985), pp. 245-257.
The phylogeny is extremely similar to Stirton’s through Parahippus, but the upper branches are a bit more detailed. Instead of having the genus Equus be a descendant of Pliohippus, Pliohippus is relegated to an offshoot that goes extinct before the Pliocene, the genus Dinohippus giving rise to Equus and the recent horses of the New and Old World in that genera. We will come back to the work of MacFadden later, but it is important to note how close the ideas of researchers in decades past were with modern understanding in this area.

From Quinn, J. H. 1955. Miocene Equidae of the Texas Gulf Coastal Plain. Bur. Econ. Geol., Univ. Texas Pub. 5516: 102 pp. (Click for larger image)
J.H. Quinn’s 1955 phylogeny of the horses of the Texas Gulf Coastal Plain was even more wildly branching than Stirton’s, and while Quinn’s focus was a bit more narrow, the tree is much more divergent. Other researchers had the genus Equus arising in the late Pliocene (and even as late as the Pleistocene), Quinn’s version has Equus appearing as early as the middle Miocene, Merychippus, again being nominated as the progenitor of all the subsequent forms in the area. While this version of horse evolution has been extensively reshuffled and revised, it is important to note that the idea that horses evolved in a straight line was well out of fashion by the middle of the 20th century at the very latest. Why, then, did it hang on for so long in the public mind?

A mount of Mercyhippus isonesus quintus. From Simpson, George Gaylord. 1932. “Mounted skeletons of Eohippus, Merychippus, and Hesperosiren.” American Museum novitates ; no. 587
Part of the problem with museums is that it takes a lot of time, money, and effort to revise exhibitions, and for some time the American Museum of Natural History horse display (THE display that illustrated horse evolution for many years) followed a progression like that of W.D. Matthew’s simplified diagram (see above). While this was eventually changed when the fossil halls were refurbished, it still seemed to show a straight line of descent, and even the display that stands on the fourth floor of the museum today reflects such a transition. If you read the plaques and take the time to compare the skeletons the branching nature of horse evolution is apparent, but the fossils themselves are arranged from Eohippus to Equus in a two parallel straight lines, showing an overall smaller-to-larger and many-to-one toe progression. Likewise, popular books on evolution and paleontology seemed hard-pressed to let go of straight-line evidence. While it could be said that such books were correct in that we could follow the line of descent from modern horses backwards to the exclusion of other groups, this approach seems to do more harm than good in the long run. Take A.S. Romer’s Man and the Vertebrates: Vol. I, for example. Originally published in 1933, my 1954 Pelican Books paperback edition shows the fossil limbs of Eohippus, Miohippus, Merychippus, and Equus from left to right, once again giving the illusion of a pure line of anagenesis. No hint of a larger diversity is given outside a brief mention of the modern forms of Zebra, Ass, and Prezwalski’s Horse.

Comparison of Eohippus to Equus. There’s a lot of evolution in that dashed line. From “The Dawn Horse or Eohippus” by Chester Stock (1947).
The 1966 edition of Romer’s Vertebrate Paleontology fairs better overall, but is still found wanting. The same straight-line illustration I just mentioned is found in the section treating perissodactyls as a group, and the skeletons of Eohippus, Mesohippus, and Hippidion are shown left to right across pages 266 and 267. While the text does mention an overall diversity of forms, as well as using certain genera for the “type” from which modern horses evolved, the overall visual impression of simple anagenesis remains. Again, I doubt the casual reader picked up Romer’s book for light nightly reading, but it is strange that the progressive ideas about evolution during that time are so poorly represented.
A similar time-capsule is Edwin Colbert’s Evolution of the Vertebrates, originally printed in 1955. The 1966 edition is the one that I acquired, and it is an interesting contrast to Romer’s book. At first Colbert seems to fall into the same trappings of straight-line evolution, showing a simple progression (in text with arrows) from Hyracotherium (Eohippus) -> Orohippus ->Epihippus -> Mesohippus -> Miohippus, spanning the Lower Eocene to the Upper Oligocene. After this progression, however, Colbert does note that there was a proliferation in forms;
By the end of the Oligocene epoch the horses had through these changes attained the status of advanced browsers, capable of eating leaves and soft plants and able to run fairly rapidly and for sustained periods over hard ground. With the advent of Miocene times there was a branching out of horses along several lines of development, probably as a response to an increase in the variety of environments available to them, and especially because of the spread of early grasses and other flowering plants.
An illustration on page 364 makes something of an attempt to reflect this visually, following the phylogeny of R.A. Stirton (see above) but in a more subdued and compressed manner. Being that only the genus names are mentioned, Colbert’s tree looks especially bare, although it must be conceded that it is a more accurate depiction of horse evolution than Romer’s. The illustration on page 148 of the 1961 paperback edition of G.G. Simpson’s Horses more closely follows Stirton’s phylogeny as well, and the plates likewise show the branching of tooth shapes and other characters rather than grouping forms separated by large expanses of time. The relatively rich fossil record of horses would be important to Simpson in another way as well; in his Neo-Darwinian Synthesis-era work, Tempo and Mode in Evolution (1944), Simpson was able to conclude that horses in general seemed to evolve faster than unrelated groups of animals like ammonites but more slowly than mammals like elephants. Although his hypothesis of a near-constant, albeit accelerated, rate for horse evolution has not held up today, the idea that evolution can occur more quickly or more slowly was a very important idea, an idea that took new form in Eldredge & Gould’s hypothesis of punctuated equilibria decades later.

From McFadden, Bruce. 2005. “Fossil Horses – Evidence of Evolution.” Science Vol. 307. no. 5716, pp. 1728 – 1730
So what of our current understanding of horse evolution? As I had mentioned earlier, one of the foremost authorities on the topic is Bruce MacFadden, and in 2005 he authored a straightforward summation of the current state of things in an article entitled “Fossil Horses – Evidence for Evolution.” As MacFadden notes, the overall “look” for the tree, featuring lines that did not leave modern descendants, hasn’t changed much since the time of Stirton and other earlier scientists. There has been much shuffling around and plenty of new discoveries, however, and although the diversity of late horses often gets the most attention it is now being revealed that early members of the horse lineage had a wider diversity as well. It almost seems like there’s an evolutionary bottleneck during the Oligocene, with the beginnings of more diversity in the Miocene, Mercyhippus once again leading the charge on to later forms.
MacFadden also takes a moment to correct a common misconception about horse evolution; there was no unalterable progression from small to large consonant with Cope’s Rule;
Although the 55-My old fossil horse sequence has been used as a classic example of Cope’s rule, this notion is now known to be incorrect. Rather than a linear progression toward larger body size, fossil horse macroevolution is characterized by two distinctly different phases. From 55 to 20 Ma, primitive horses had estimated body sizes between ~10 and 50 kg. In contrast, from 20 Ma until the present, fossil horses were more diverse in their body sizes. Some clades became larger (like those that gave rise to Equus), others remained relatively static in body size, and others became smaller over time.
Still, our current understanding is incomplete, and further fossil finds will continue to rustle the branches of the evolutionary bush. In fact, I would not be surprised if more early forms came to light, and I would be especially interested to see if the “Oligocene Bottleneck” is real or merely a factor of fossil collecting bias. There should be no mistake about the amazing entanglement of branches horses represent, however, and it is somewhat surprising that the public does not often hear about the true form of horse phylogeny. While I did not do an in-depth study of how horse evolution was portrayed in the popular media, from what I have seen it seems that past scientists and authors have often opted for simplicity, getting the public to accept evolution has occurred being more important than giving them an accurate depiction of how evolution works. This is a harsh lesson that we are still learning, as inaccuracies in books, museum displays, and other outlets can leave the door open for creationists to spread distrust of science. It is not enough to merely present someone who is unfamiliar with evolution with our “best” example of anagenesis; if we do, it should be in context with the larger theme of unity and diversity of forms, not a throw-away that is supposed to dazzle in and of itself. The evolution of the horse, in fact, is a perfect example of evolution and can be an extremely powerful tool in education if used properly, but for whatever reason the common theme so far has been for many popular science writers and educators to fall out of the saddle.
Evolution is truly an amazing phenomenon; who would have ever conceived of a small, four-toed animal giving rise to Black Beauty? Our overall conception of “more” being better may even make such a move from four toes to one seem counterintuitive, yet the evidence (from fossils to that of development) is clear in its implications. Horses did not spring up out of the ground from the dust, nor were they “spoken into being” by an omnipotent power that decided that Adam should have a faithful steed. Every bone in their body cries out as to their past, and we are all the more enriched to understand that just like the horse, Homo sapiens is a still-changing product of a long and rich evolutionary history, too.
































Recent Comments